Translate this page into:
A case report on rare hepatitis B viral subgenotype from a tertiary care center in Chennai
*Corresponding author: Padma Srikanth, Department of Microbiololgy, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India. padmasrikanth@sriramachandra.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Mani M, Gnanaskandan S, Vijayaraghavan S, Srikanth P. A case report on rare hepatitis B viral subgenotype from a tertiary care center in Chennai. Sri Ramachandra J Health Sci 2021;1:22-4.
Abstract
Hepatitis B virus (HBV) is a global health concern with 350 million chronic carriers. With respect to HBV India is classified as an intermediate endemic country. The disease progression may be due to many viral factors including HBV viral load, HBe antigen, genotype, mutations in polymerase gene, and X gene. In this case, the individual was a treatment naïve chronic HBV carrier. The reverse transcriptase gene and X gene were sequenced and mutations were analyzed. The individual had D3 subgenotype. rt80I was identified in reverse transcriptase and A102V in HBx protein. Identification of genotype and mutations in reverse transcriptase/X gene may help in predicting and improving the clinical outcomes.
Keywords
Hepatitis B virus
Genotype
Liver disease
X gene
Reverse transcriptase gene
INTRODUCTION
Hepatitis B Virus (HBV) related diseases such as chronic hepatitis, liver cirrhosis (LC), hepatocellular carcinoma (HCC) are on the rise and have an annual death rate of about 600,000.[1] HBV is one of the most common causes of HCC. Globally there are around 350 million chronic carriers of HBV. The prevalence of HBV in India is 2–8% and falls into the intermediate endemic category. It is a DNA virus. However, the viral replication is through reverse transcription mechanism, mimicking HIV. The DNA polymerase does not possess proofreading activity and results in the production of genetic variants.[2] HBV has ten genotypes.[3] Genotypes play a role in clinical outcome, mode of transmission, and endemicity.[4] There are few anti-viral drugs including lamivudine, telbivudine, entecavir, tenofovir, and adefovir approved by the Food and Drug Administration (FDA) that are effective against HBV. These drugs target reverse transcriptase and halt the replication activity of the virus. Each drug has their respective hotspot in the reverse transcriptase gene based on the site of action of the drug.[5] Aminoacid substitutions in the hotspot may cause drug resistance and treatment failure.
CASE REPORT
A 71-year-old male belonging to socioeconomic class –II from Chennai was admitted in our tertiary care center with complaints of abdominal pain and distention for 20 days. He was jaundiced along with a loss of weight and appetite. He described a pricking sensation in the right hypochondrium and epigastric region and had abdominal distention after food intake. There was no history of breathlessness, chest pain, or pedal edema. There were no complaints of vomiting, loose stools or melena, altered bowel habits, or any urinary complaints. No history of blood transfusion or tattooing, systemic hypertension, bronchial asthma, epilepsy, or CAD. Patient had normal sleep and appetite 20 days before admission. Patient has been smoking for the past 30 years (4-5/day) and consumes alcohol occasionally. He was afebrile, the abdomen was warm and tenderness was observed in right hypochondrium, epigastric and umbilical regions. However, organomegaly was not observed. Respiratory symptoms were insignificant, had no focal neurological deficits. Blood investigations were as follows: Hb was 10.9, platelet count 1.39, Total WBC count was 8200, and Renal Function Test was within normal limits. PT test value (28.5)/PT control (11–13.5)/INR (1.35). He was screened for viral markers and was found to be seropositive for HBV surface antigen by ELISA and seronegative for Human Immunodeficiency Virus-1 and Hepatitis C Virus. Liver Function Tests were abnormal. Total bilirubin 9.92, direct bilirubin 5.21, SGOT 642, SGPT 223. Hepatitis B e antigen, the replicative marker was found to be positive.
Ultrasound of the abdomen was done and it showed coarse echotexture of the liver with nodular margins and minimal ascites with thickened gall bladder wall secondary to Ascites [Figure 1]. Hence he was diagnosed with Hepatitis B-related decompensated liver disease, as he was reactive to HBsAg, LFT was repeated, and elevated levels were found. HBV quantitative real-time polymerase chain reaction (PCR) was performed using Artus HBV RG PCR kit (Qiagen, Hilden, Germany) in rotor gene Q PCR system and HBV DNA load was 116835 IU/ML. The patient has signed the inform consent and HBV genotyping was performed targeting the reverse transcriptase gene. Around 1300bp of the gene including YMDD motif which is a catalytic site was sequenced using four specific overlapping primers in ABI 3730 platform. The sequences were retrieved and consensus was submitted to HBVseq HIV drug resistance database (https://hivdb.stanford.edu) [Figure 2] and HBVgeno2pheno database (http://hbv.geno2pheno.org/). The strain belonged to genotype D and subgenotype D3. One compensatory mutation rt80I was identified in the reverse transcriptase which is related to lamivudine and telbivudine resistance. The patient was treated with 0.5 mg entecavir and showed signs of improvement. The X gene was amplified and sequenced as previously published[6] for mutations, we found seven mutations in which one was significant A102V which was earlier reported in individuals with LC.[7]
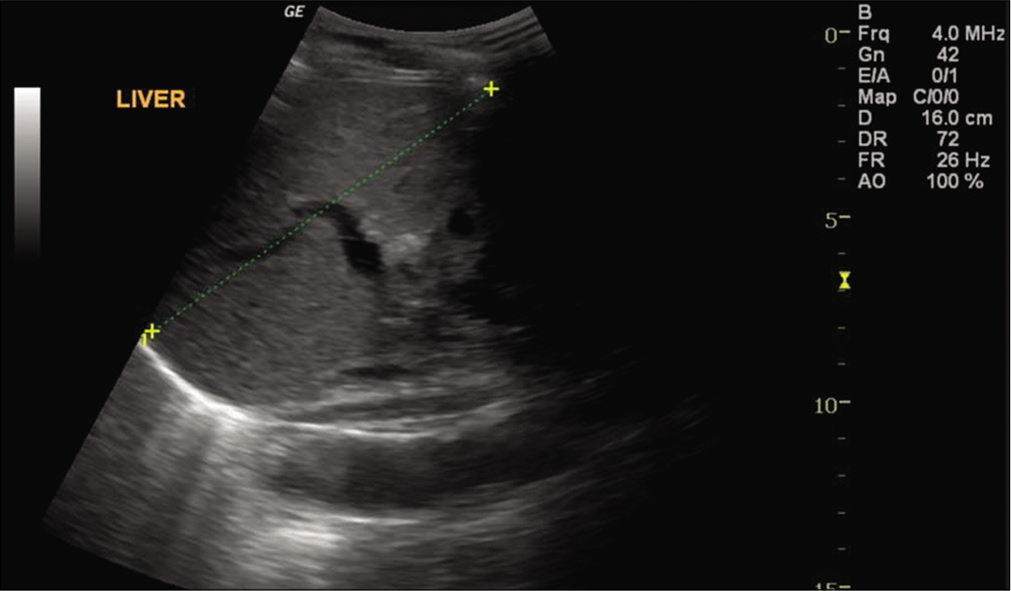
- Ultrasound image of liver showing echo texture and nodular margin.
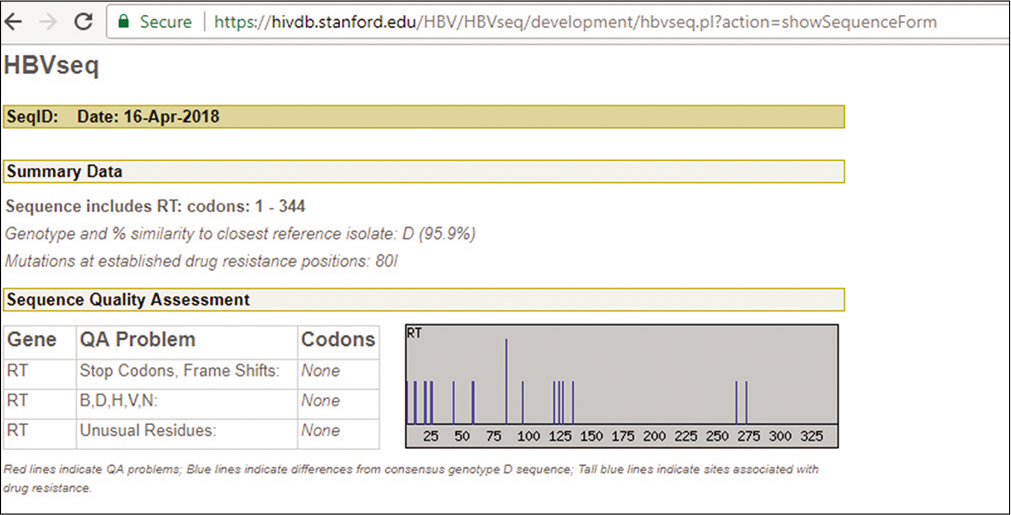
- Snapshot of report from database (hivdb.stanford. edu/HBV/HBVseq) after submitting hepatitis B virus reverse transcriptase sequence, tall line indicates compensatory mutation rtL80V in the participant.
DISCUSSION
In this case, the patient was not treated with HBV anti-viral drugs. HBV e antigen, which is a marker of active replication, demonstrated a positive correlation with the viral load.[8] Genotypes and subgenotypes of HBV are geographically distinct.[9] The predominant genotype in India is D, followed by A and C. Genotype D has nine subgenotypes.[10] Globally, there are 600,000 deaths annually, related to HBV-related diseases, such as chronic hepatitis B (CHB), LC, and HCC. Many viral factors such as viral load, genotype, and specific viral mutations, are known to affect disease progression. Genotypes have distinct characteristics; For example, genotype A has a tendency for chronicity, whereas viral mutations are frequently encountered in genotype C. Both chronicity and mutation frequency are common in genotype D.[11] LC and progression to HCC are more commonly encountered with genotypes C and D than the other genotypes. Pathogenic differences between HBV genotypes explain disease intensity, progression to LC, and HCC. Finally, genotype determination in CHB infection is important in estimating disease progression and planning optimal antiviral treatment. In Southern India, genotype D/D2 has a reported prevalence of 79%, while genotype D/D3 was rarely reported.[8,12] Antiviral resistance is a major challenge in the current HBV treatment as there are only two drugs such as Entecavir and tenofovir are currently available.
The reverse transcriptase sequences revealed that the individual was infected with genotype D and sub genotype D3. D3 has been primarily reported in Indonesia, Brazil, North America, and Europe.[13] In India, D3 was reported in small numbers in the eastern parts of the country.[14] This patient had not traveled to the places where D3 is prevalent. Patients infected with HBV genotype D will develop LC and HCC and the frequency of mutation is reported to be higher.[15] We have noted rtL80I mutation in reverse transcriptase in this case, which is one of six predominant mutations in HBV,[16] however L180M and M204V, the primary drug resistance mutations to lamivudine and telbivudine were not detected in the participant. rtL80I is a secondary or compensatory mutation to lamivudine and telbivudine. This can confer potential resistance to these drugs in the presence of other mutations like rtL180M and rt204V in domain B of reverse transcriptase. Seven mutations including A102V in × protein were identified. However, there were no mutations observed at positions 5, 130, 131 which are NFĸB activation sites in the × protein. These sites up-regulate the viral replication and lead to HCC.
CONCLUSION
The disease progression of HBV is genotype-specific. Since genotypes were geographically restricted, clinicians do not request for genotype testing routinely under the assumption that genotypes were geographically restricted. Furthermore, drug resistance reported in certain genotypes. There, the need for genotyping not thought to be important unless the patients has persistent high viral load. Determining genotype and drug-resistant mutations in reverse transcriptase gene prior to treatment is significant in assessing the disease progression and plan a strategy for customized anti-viral therapy. X gene mutations can be a molecular marker for predicting the clinical outcomes of chronic HBV carriers.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The Global Burden of Viral Hepatitis: Better Estimates to Guide Hepatitis Elimination Efforts. 2019. World Health Organization. Available from: http://www.who.int/mediacentre/commentaries/better-estimates-hepatitis/en [Last accessed on 2019 Jan 12]
- [Google Scholar]
- Overview of hepatitis B virus mutations and their implications in the management of infection. World J Gastroenterol. 2016;22:145-54.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic diversity of hepatitis B virus strains derived worldwide: Genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47:289-309.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med. 2015;5:a021436.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatitis B Virus genotypic differences map structurally close to NRTI resistance hot spots. Int J Curr Chem. 2011;2:253-60.
- [Google Scholar]
- Molecular characterization of hepatitis B virus X gene in chronic hepatitis B patients. Virol J. 2012;9:131.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of hepatitis B virus (HBV) X gene mutations on hepatocellular carcinoma development in chronic HBV infection. Clin Vaccine Immunol. 2011;18:914-21.
- [CrossRef] [PubMed] [Google Scholar]
- Antiviral resistance mutations and genotype-associated amino acid substitutions in treatment-naïve hepatitis B virus-infected individuals from the Indian subcontinent. Intervirology. 2012;55:36-44.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatitis B virus genotypes and genome characteristics in China. World J Gastroenterol. 2015;21:6684-97.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatitis B virus genotypes: Global distribution and clinical importance. World J Gastroenterol. 2014;20:5427-34.
- [CrossRef] [PubMed] [Google Scholar]
- HBV genotypes in India: Do they influence disease severity? Hepatol Res. 2009;39:157-63.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of hepatitis B virus genotypes in blood donors and chronically infected patients in a tertiary care hospital in Southern India. Clin Infect Dis. 2004;38:e81-6.
- [CrossRef] [PubMed] [Google Scholar]
- Full-genome sequences of hepatitis B virus subgenotype D3 isolates from the Brazilian Amazon Region. Mem Inst Oswaldo Cruz. 2015;110:151-3.
- [CrossRef] [PubMed] [Google Scholar]
- An overview of molecular epidemiology of hepatitis B virus (HBV) in India. Virol J. 2008;5:156.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatol Int. 2008;3:334-42.
- [CrossRef] [PubMed] [Google Scholar]
- Naturally occurring hepatitis B virus reverse transcriptase mutations related to potential antiviral drug resistance and liver disease progression. World J Gastroenterol. 2018;24:1708-24.
- [CrossRef] [PubMed] [Google Scholar]






