Translate this page into:
Granulomatosis with polyangiitis masquerading as disseminated tuberculosis in presence of bilateral lung cavities and hemorrhagic infarct
*Corresponding author: Shital Patil, Department of Pulmonary Medicine, Pacific Institute of Medical Sciences Udaipur, Udaipur, Rajasthan, India. drsvpatil1980@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Patil S, Tak S. Granulomatosis with polyangiitis masquerading as disseminated tuberculosis in presence of bilateral lung cavities and hemorrhagic infarct. Sri Ramachandra J Health Sci. 2024;4:26-30. doi: 10.25259/SRJHS_28_2022
Abstract
Lung cavitations with constitutional symptoms are usually associated with pulmonary tuberculosis due to its high prevalence and are the most common infectious cause in India. Granulomatosis with polyangiitis (GPA) with the systemic pulmonary disease will have different radiological presentations such as nodule, mass, and consolidation with or without cavitation. In this case report, we have reported a 40-year-old female presented with cough, fever, and weight loss of 6 months duration with chest imaging suggestive of bilateral cavitary lung disease. She was labeled as a case of disseminated tuberculosis due to involvement of the central nervous system and empirically treated with anti-tuberculosis treatment (ATT) without microbiological evidence of tuberculosis in smear and cartridge-based nucleic acid amplification tests. The absence of clinical response and radiological worsening to empirical ATT gives a clue for an alternate diagnosis. Bronchoscopy workup documented Aspergillus colonization and vasculitis workup in the presence of cavitary lung disease documented abnormally raised proteinase 3 anti-neutrophil cytoplasmic antibodies titers. She was treated with a combination of systemic methylprednisolone and azathioprine with voriconazole. Her clinical response was observed in 4 weeks and radiological response with complete radiological clearance of pulmonary cavitations was documented after 6 months of treatment. We recommend prompt workup and ruling out GPA in all cases with pulmonary cavitations with negative microbiological workup and no empirical ATT should be given in the era of rapid microbiological tests with good sensitivity and specificity.
Keywords
Cavitating lung disease
High-resolution computed tomography (HRCT) thorax
Papilledema with hemorrhagic infarct
Granulomatosis with polyangiitis
Pulmonary tuberculosis
INTRODUCTION
Granulomatosis with polyangiitis (GPA) previously called as Wegener’s granulomatosis, is systemic vasculitis involving small and medium-sized vessels was first described by a German medical student in 1931 and, later German pathologist Friedrich Wegener in 1936 described the case series of “distinct form of vasculitis” in three middle age patients with small and or medium vessel vasculitis with granulomatous inflammation. Goldman and Churg, in 1954, published a review of 22 cases with this distinct small and medium vessel vasculitis with granulomatous inflammation defined as Wegener’s granulomatosis.[1] In 2012, the classification of the modern nomenclature of systemic vasculitis was formulated at the Chapel Hill consensus conference, and Wegener’s granulomatosis was changed to GPA.[2] Due to diverse radiological presentations, the majority of Wegner’s granulomatosis or GPA are usually misdiagnosed, and the majority of these cases receive empirical treatment. In tropical settings, GPA is misdiagnosed and usually treated as tuberculosis. In our case, we have observed that empirical anti-tuberculosis treatment (ATT) was offered in the presence of cavitating lung disease with eye and central nervous system (CNS) involvement which is misdiagnosed as disseminated tuberculosis.
CASE REPORT
In the present case, a 40-year-old female patient referred by her family physician for ATT-induced liver dysfunction and dehydration-induced renal dysfunction in the case of pulmonary tuberculosis suspect treated with empirical ATT. She is a farmer and housewife without any addictions and comorbidities, having constitutional symptoms such as fever, cough, and weight loss for 6 months. She was treated with oral and intravenous antibiotics and hospitalized in peripheral health centers. Her illness gradually worsened with a partial response to medical treatment. Due to cavitary lung disease in chest X-ray, she was labeled as a case of pulmonary tuberculosis without microbiological confirmation and documentation of mycobacterium tuberculosis (MTB) genome in cartridge-based nucleic acid amplification tests (CBNAAT) and negative sputum smear microscopy. She was treated with empirical ATT for 4 months and referred to our center for ATT-induced hepatotoxicity and dehydration due to her inability to tolerate anything by mouth. We have done high-resolution computed tomography (HRCT) thorax and documented bilateral, peripheral multifocal consolidations involving middle and lower lobes with air bronchogram consolidation with lucencies [Figure 1]. Cerebrospinal fluid (CSF) analysis done at the neurology center due to headache and revealed normal biochemistry, lymphocytosis with 10 cells, adenosine deaminase level 6 IU/L (normal range 0–10 IU/liter), and microbiological examination negative from Gram stain and Ziehl–Nelson stain. CSF CBNAAT was negative for the MTB genome. Her fundoscopic examination findings records revealed papilledema with optic atrophy due to chronic papilledema [Figure 2]. Both eyes’ fundus examination documented disc edema, cup full, pallor present, and gross vascular attenuation [Figure 3]. Magnetic resonance imaging brain imaging documented subacute non-hemorrhagic infarct right occipital lobe and deep microvascular ischemic changes [Figure 4]. Retrospectively, the patient narrated a history of crusting in the nose and throat with intermittent altered speech for 2 years.
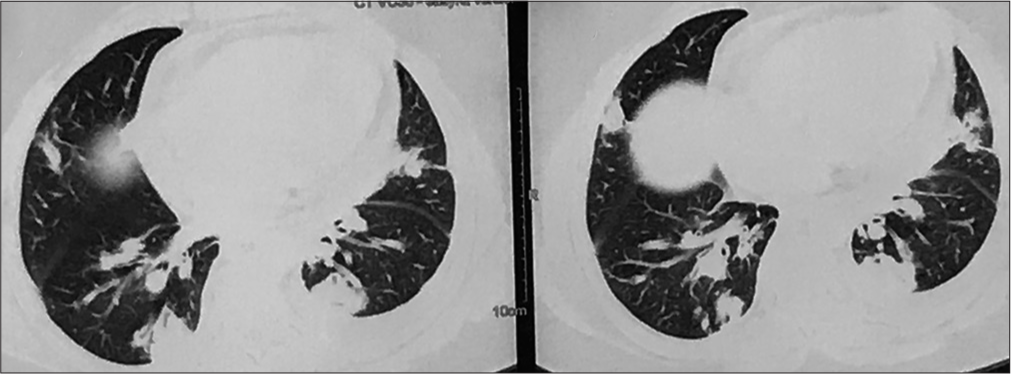
- High-resolution computed tomography (HRCT) thorax showing bilateral, peripheral consolidation.
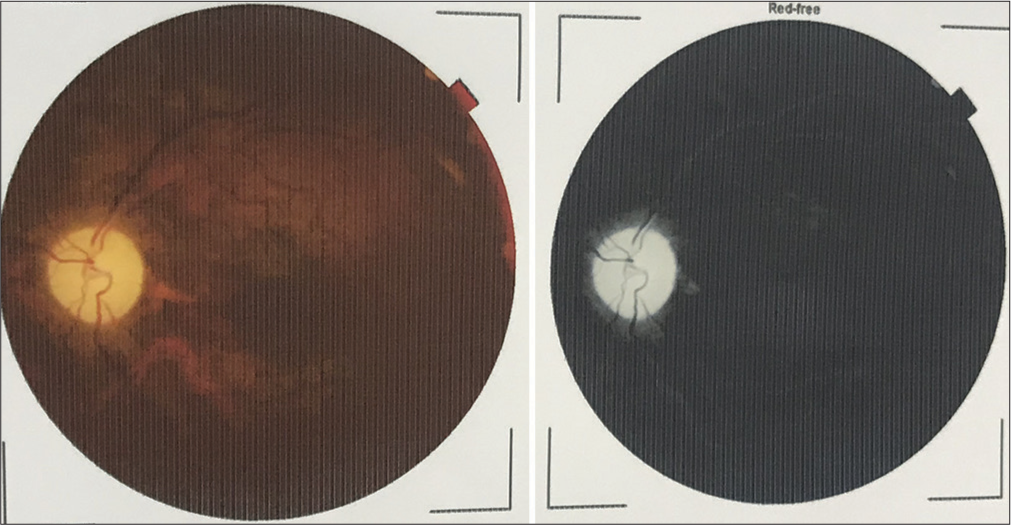
- Fundoscopic examination showing papilledema with optic atrophy.
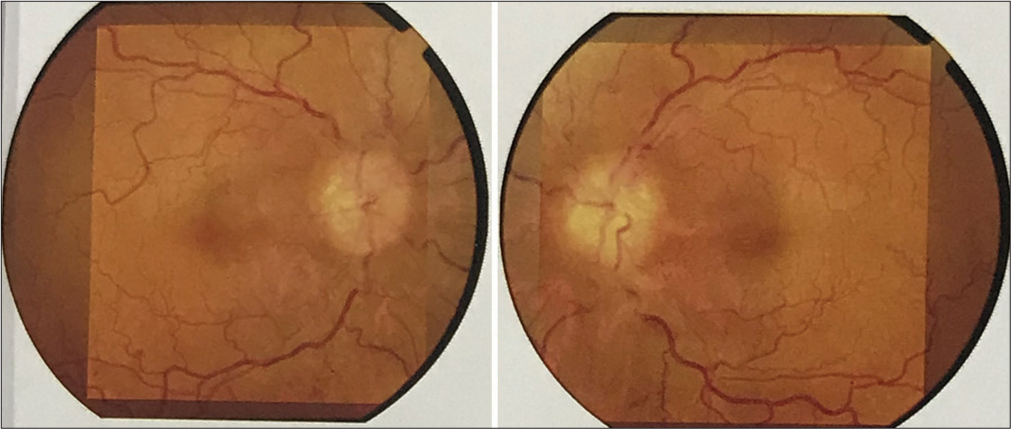
- Fundus examination showing optic disc edema, optic cup full, and pallor present with gross vascular attenuation.
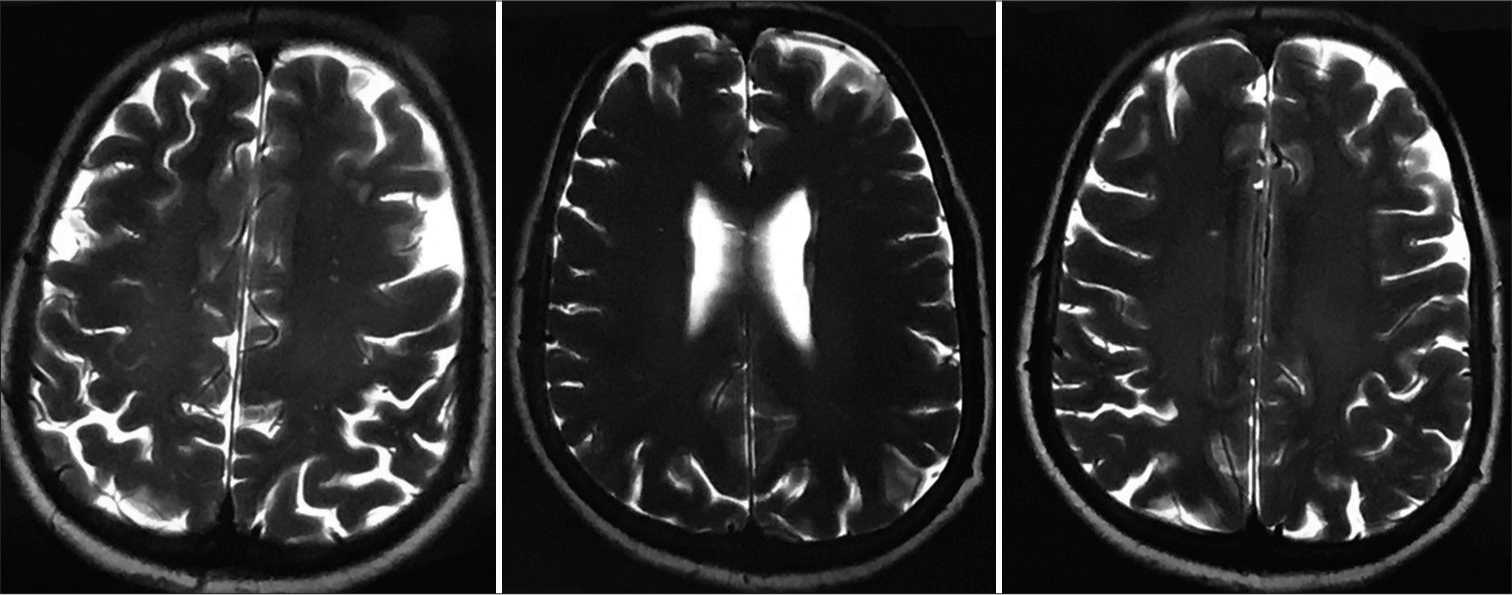
- Magnetic resonance imaging brain imaging showing subacute non-hemorrhagic infarct right occipital lobe and deep microvascular ischemic changes.
During hospitalization, ATT was withheld or omitted and supportive treatment was started for renal and liver dysfunction with targets for maintenance of hemodynamic parameters such as blood pressure, urine output, and respiratory rate. Her general condition and liver functions improved. We have repeated HRCT thorax documented bilateral pulmonary cavities, randomly placed, bizarre-shaped, irregular, thin, and thick-walled at some point without fluid level. A few cavities are giant, stepladder appearance, and variegated appearance. Some cavities contain radio-opaque mass-like structures attached to one side wall, possibility of mycetoma [Figure 5].
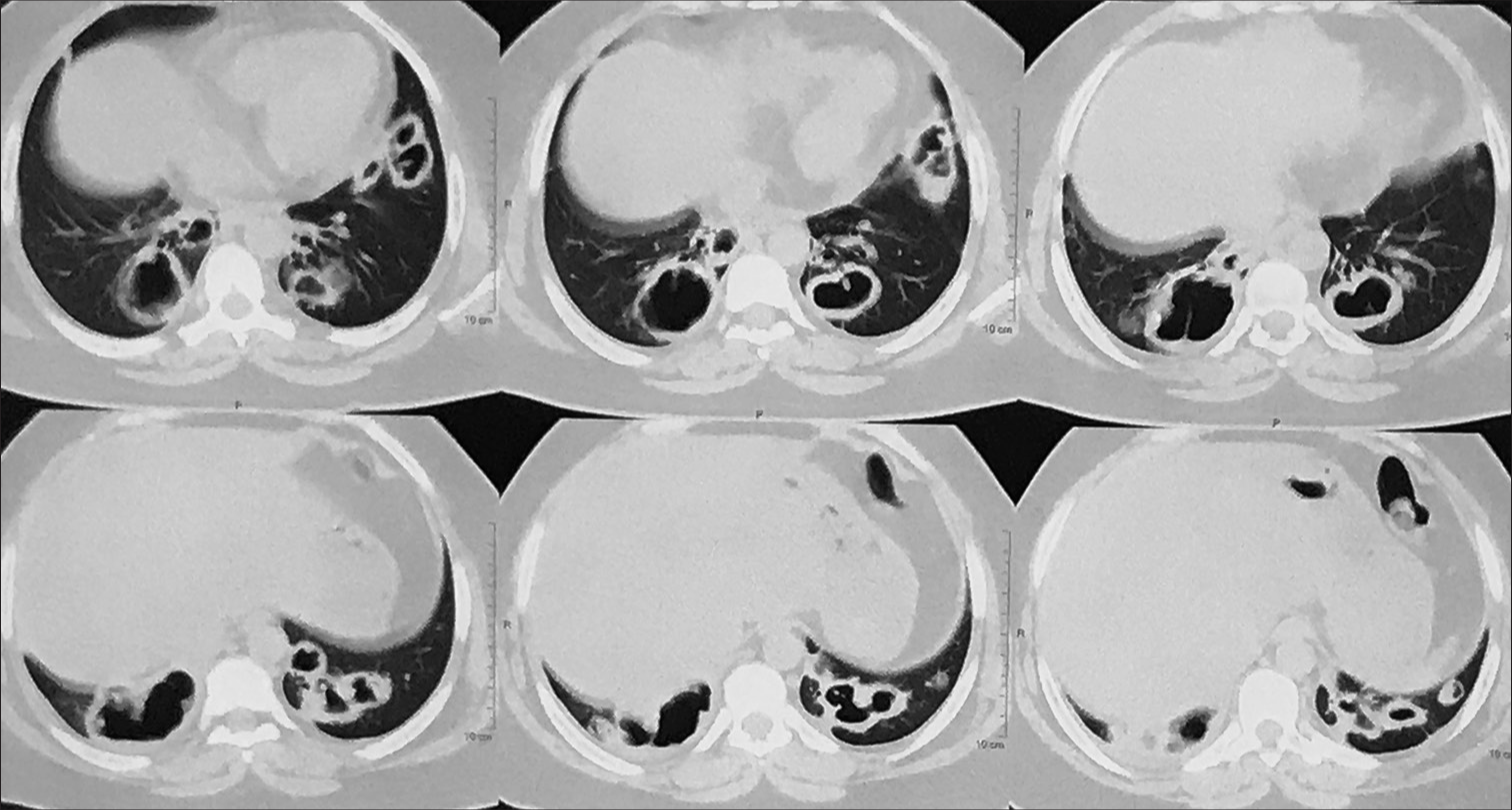
- High-resolution computed tomography (HRCT) thorax bilateral peripheral, pleural-based thick-walled, irregular, variegated, and bizarre-shaped cavities with intracavitary opacities.
A bronchoscopy was performed for a definitive diagnosis of cavitary lung disease with a negative microbiological workup. During bronchoscopy, an abnormality was documented in tracheobronchial lumens from the mainstem to segmental bronchial areas with abundant mucopurulent secretions coming out from the bronchial lumen was collected in three different aliquots and sent for bacterial, fungal, and malignancy. Bacterial, and tuberculosis workup were found inconclusive with negative CBNAAT results. We have done vasculitis workup in the presence of typical radiological patterns and bizarre-shaped peripheral lung cavities to rule out GPA. Vasculitis workup showed myeloperoxidase antineutrophil cytoplasmic antibody (ANCA) (P-ANCA) 0.92 RU/mL (normal range 0–20 RU/mL) and proteinase 3-ANCA or C-ANCA was >280 RU/mL (normal range 0–20 RU/mL).
During hospitalization, after clinical stability and general health improvement, we initiated acute GPA management protocol and started injection of Methyl prednisolone (MPS) 40 mg intravenously 3 times for 7 days, a tablet of azathioprine 50 mg 1 time daily. During the 2nd week, azathioprine dose was increased to 100 mg for 12 weeks. Other supportive care, intravenous fluids, oral liquids, and antipyretics were given as a hospital protocol to maintain hemodynamic stability. Oral methylprednisolone tablet 48 mg was given in three divided doses, and the injection of methylprednisolone was stopped. Protocolized tapering of methylprednisolone was done over 12 weeks as a 4 mg reduction in every 1 week and a dose of 4 mg was maintained afterward. Additional treatment with Trimethoprim Sulfamethoxazole (160/800) was given as two times daily for the one month for the prevention and treatment of secondary opportunistic infections in GPA induced pulmonary cavities due to disease related and steroid induced immunosuppression. After 1 month, trimethoprim sulfamethoxazole (160/800) was given 1 time daily for3 months and decreased to 1 time per week for 2 years. We have monitored hemogram, liver, and kidney functions weekly, then monthly as a protocol to assess further steroid, and azathioprine-induced organ dysfunction.
Antifungal tablet voriconazole 200 mg 2 times daily was given for 12 weeks for resolution of fungal colonization in pulmonary cavities. We have documented complete resolution of radiological abnormality including pulmonary cavities and fungal colonization in followup HRCT thorax with satisfactory clinical response [Figure 6].
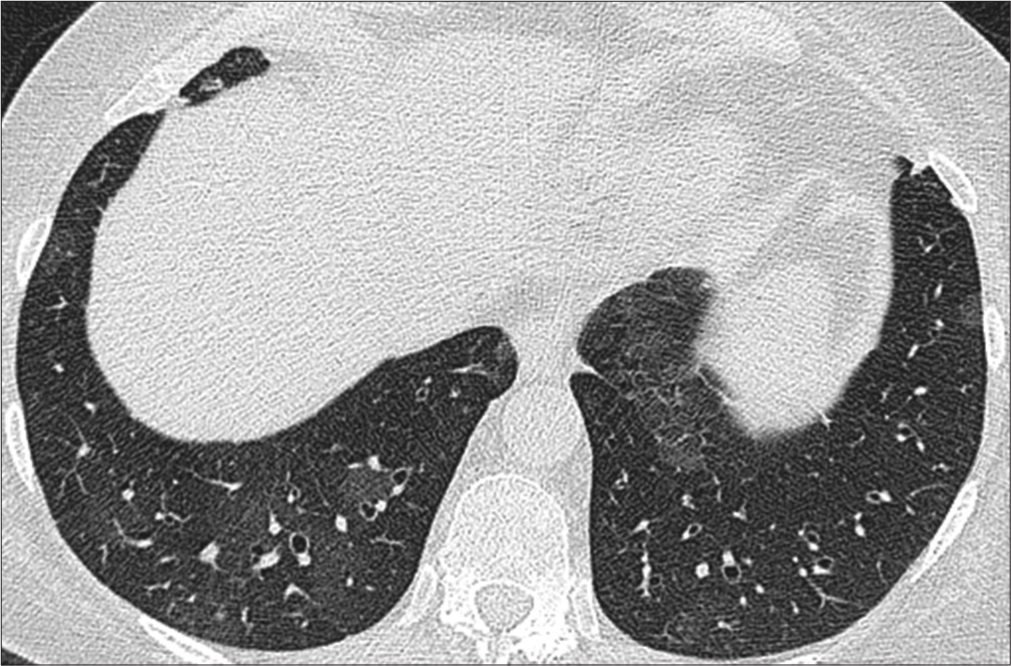
- High-resolution computed tomography (HRCT) thorax normal lung parenchyma with resolution of lung parenchymal abnormalities.
We have documented complete remission of GPA during maintenance of azathioprine 100 mg 1 time daily for 2 years with monitoring of complete hemogram, liver, and kidney functions monthly.
DISCUSSION
GPA is characterized by small and medium-sized vessel vasculitis, also called as a pulmonary-renal syndrome, with predominant otorhinolaryngological complications. The exact etiology of this multisystem disease is unknown. The possible link of ANCA was precluded in the pathophysiology of GPA. ANCA is a plausible pathway for inflammatory insult documented in GPA and complex interactions between microbial proteins and host genetics would have been documented to increase susceptibility and occurrence in selected cases. In addition, ANCA activates the proliferation of neutrophils toward the endothelium of small and mediumsized vessels with abnormal degranulation of neutrophils will cause endotheliitis and microabscesses and finally, ill-defined or poorly formed granulomas will form, which has been very well documented in histopathology.[3]
Possible pathophysiological links in ANCAs associated with GPA in published literature
ANCAs have been documented with inflammatory conditions such as rheumatoid arthritis and systemic lupus erythematosus and malignant conditions such as Hodgkin’s lymphoma and myelodysplasia. Antigenic cross-reactivity induced by connective tissue proteins, tumor antigens, and host immune cells is the possible mechanism for ANCA.[4,5]
ANCAs have been documented with inflammatory conditions such as staphylococcus aureus infection and GPA and other infections such as hepatitis C virus, cytomegalovirus, Epstein–Barr virus, and parvovirus also reported possible coexistence of ANCAs. Robust data are available regarding Staphylococcus colonization in nasal sinuses and skin; and the occurrence of GPA. The plausible mechanism would be staphylococcus-related super-antigens with host interactions, which have resulted in exaggerated inflammatory pathway and ANCA.[6]
ANCAs have also been documented with various drugs such as levamisole, adulterated cocaine, and other drugs such as hydralazine, propylthiouracil, minocycline, phenytoin, antithyroid medications, sulfasalazine, and allopurinol have been reported to be associated as drug-induced host interactions and exogenous antigen-induced inflammatory pathway activation.[7]
Granuloma in GPA
Granulomas in GPAs were seen as well-defined to ill-defined and poorly formed architecture of inflammatory cells and are associated with neutrophilic microabscesses. These granulomas are initiated with ANCA-associated vasculitis, resulting in the accumulation of inflammatory cells such as plasma cells, lymphocytes, and dendritic cells, leading to partial or near total occlusion of small- to mediumsized vessels. These granulomas are different from those documented with sarcoidosis and tuberculosis regarding the pattern of inflammatory cells and accompanied giant cells in these granulomas as reported in histological examination, which will cause necrotic damage to cartilage, bone, and soft tissues due to vasculitis and microthrombosis-related hypoxic and cytotoxic inflammatory pathways.[8]
Nodules, consolidation, and cavitation in GPA
Pulmonary involvement in GPAs is described as nodules, masses with or without cavitation. Nodules and masses are usually multiple, peripheral, pleural based, randomly placed, and having propensity to caveated in almost one-fourth cases due to vasculitis-related necrosis. Cavities are usually missed due to infections such as tuberculosis, septic infarcts, lung abscesses, and malignancies due to their appearance and overlapping radiological picture.[9] The majority of GPAs with pulmonary cavitations usually missed as tuberculosis due to more prevalent nature and cavitations, which are sometimes showing secondary infections and fluid levels, giving a typical tuberculous cavity radiological picture. In addition, cavitations in GPAs have a propensity for secondary fungal colonization, which will present such as mycetoma or mass with cavitation. Importantly, cavitation in GPAs has irregular marines and thick walls which are a differentiating feature from other etiologies as described. Authors have published cases of Wegener’s misdiagnosed as tuberculosis in overlapping clinical and radiological pictures masquerading as ongoing Wegener’s disease or GPA.[10-13]
CONCLUSION
In the present case report, we have observed clinical scenarios in GPA misdiagnosed as cavitary lung disease due to tuberculosis with CNS dissemination and failed to show clinical and radiological response to ATT. Recurrence of symptoms with the withdrawal of steroids and bronchoscopy-guided workup ruled out tuberculosis with superadded cavitary fungal infection. Vasculitis workup established GPA with systemic involvement and documented successful treatment outcome after treatment with methylprednisolone, azathioprine, and voriconazole with complete remission and radiological resolution of lung lesions.
Key learning points from this case report are:
Although pulmonary tuberculosis is the most common cause of pulmonary cavities with constitutional symptoms, a microbiological workup is recommended for documentation of acid-fast bacilli in the smear or genome in CBNAAT, later being the most sensitive and specific test for confirmations of tuberculosis. No empirical ATT should be given in the era of rapid molecular-based diagnostic techniques available, as; it will give the correct diagnosis and it will guide for drug-sensitive and drug-resistant tuberculosis as well.
Differentials for cavitary lung disease are lung abscess, septic infarcts, cavitation GPAs, and fungal infections. Typical radiological features for GPAs are bizarre, irregularly shaped, and; usually bilateral, and multiple pleural-based cavities with irregular and thick margins will differentiate it from other causes.
GPAs with pulmonary involvement and mimicking as tuberculosis are not uncommon, it should be suspected in the presence of constitutional symptoms and cavitation with negative microbiological workup because microbiological workup will have the highest microbiological yield in cavitary disease, and negative will give a clue for an alternate diagnosis.
Disseminated tuberculosis is the involvement of more than two non-continuous organ systems due to the tuberculosis process or the involvement of blood or bone marrow due to the tuberculous process either due to lymphatic, hematogenous, or lymphohematogenous dissemination of tuberculous bacilli. Pulmonary cavities with hemorrhagic infarct with papilledema are possible in disseminated tuberculosis as observed in our case, but is unlikely the etiology in the presence of negative microbiological workup with no response to empirical ATT for 4 months.
Other ancillary features such as sinusitis, recurrent rhinitis, and nasal crusting give clinical clues to workup in line with GPA as documented in our case apart from clinical radiological worsening to routine treatment in suspected cases of tropical infections such as tuberculosis.
We recommend a workup for GPA in cases with cavitary lung disease with constitutional symptoms and upper airway symptoms such as nasal crusting and altered speech. GPA has shown excellent clinical and radiological response to medical treatment options such as methylprednisolone and azathioprine combinations with successful remission for a longer period. Voriconazole is the drug of choice for pulmonary secondary fungal colorization cavities due to GPA or healed TB cavities. Generally antifungal is not recommended for use in cases with mycetoma. In settings with chances of invasive, fungal infections are high in cases with mycetoma and having underlying comorbidity or cases receiving systemic steroids as in our case.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Wegener’s granulomatosis: Pathology and review of the literature. AMA Arch Pathol. 1954;58:533-53.
- [Google Scholar]
- 2012 revised International Chapel Hill Consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of ANCA-associated vasculitis. Curr Rheumatol Rep. 2012;14:481-93.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical presentation and therapy of diseases related to anti-neutrophil cytoplasmic antibodies (ANCA) Autoimmun Rev. 2016;15:978-82.
- [CrossRef] [PubMed] [Google Scholar]
- Antineutrophilic cytoplasmic antibody-associated vasculitis and malignant hemopathies, a retrospective study of 16 cases. Joint Bone Spine. 2017;84:51-7.
- [CrossRef] [PubMed] [Google Scholar]
- Wegener's granulomatosis and parvovirus B19 infection. Arthritis Rheum. 1994;37:1707-8.
- [CrossRef] [PubMed] [Google Scholar]
- Trojan horses: Drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr Opin Rheumatol. 2014;26:42-9.
- [CrossRef] [PubMed] [Google Scholar]
- Current understanding of the pathogenesis of granulomatosis with polyangiitis (Wegener's) Expert Rev Clin Immunol. 2013;9:641-8.
- [CrossRef] [PubMed] [Google Scholar]
- Halo sign on high-resolution CT: Findings in spectrum of pulmonary diseases with pathologic correlation. J Comput Assist Tomogr. 1999;23:622-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cavitating lung disease is not always due to tuberculosis! Wegener's granulomatosis with mycetoma with deep vein thrombosis lower limb: Case report with review of literature. Electron J Gen Med. 2023;20:em425.
- [CrossRef] [Google Scholar]
- Wegener's granulomatosis mimicking like pulmonary tuberculosis and presenting as cavitating lung disease with mycetoma: A case report with review of literature. Muller J Med Sci Res. 2022;13:103-9.
- [CrossRef] [Google Scholar]
- Bronchus sign on HRCT thorax: Presenting sign of Wegener granulomatosis with lung involvement-misdiagnosed as TB in presence of acino-nodular pattern on imaging. J Assoc Chest Physicians. 2022;10:105-11.
- [CrossRef] [Google Scholar]
- Chronic febrile respiratory illness with acinonodular consolidations as presenting feature of granulomatosis with polyangiitis: A case report with review of literature. J Assoc Pulmonologist Tamilnadu. 2022;5:116-20.
- [CrossRef] [Google Scholar]







