Translate this page into:
Prevalence of intestinal parasitic infections in HIV-positive and negative groups in some selected semi-urban areas in Nasarawa state, Nigeria
*Corresponding author: Chukwuemeka Lawrence Ani, Department of Statistics, Air Force Institute of Technology, Nigeria Air Force Base, Kaduna, Nigeria. emekaani605@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Adesue GA, Ani CL, Mashood LO, Aderupatan DE, Nedu AA, Anche JY. Prevalence of intestinal parasitic infections in HIV-positive and negative groups in some selected semi-urban areas in Nasarawa state, Nigeria. Sri Ramachandra J Health Sci 2023;3:5-13.
Abstract
Objectives:
The objective of the study is to determine the occurrence of intestinal parasites among human immunodeficiency virus (HIV)-positive and negative groups.
Materials and Methods:
A case–control study was conducted in four semi-urban areas in Nasarawa State. The study enrolled 422 participants. Stool samples were collected and examined for the presence of intestinal parasites. Blood sample was collected to investigate for HIV infection.
Results:
The overall prevalence of intestinal infections among HIV-positive individuals was 37.7% (78/207). For geohelminth infections, HIV-positive individuals were more commonly infected by hookworm at a rate of 9.7% (20/207). For Protozoa infections, HIV-positive groups were identified more with Giardia lamblia, at a rate of 8.2% (17/207). The binary logistic regression showed that factors significantly associated with parasite infections among HIV-positive individuals included being under 43 years of age (odds ratio [OR] = 2.192, confidence interval [CI]: 0.082, 3.736) and subjects with diarrhea (OR = 1.521, CI: 0.112, 2.891) for geohelminth parasite. While in the case of Protozoa infections, parasitic infections are higher among study subjects with diarrhea (OR = 1.767, CI: 0.111, 3.009).
Conclusion:
HIV-positive individuals are more susceptible to coinfections with hookworm (geohelminth) and G. lamblia (Protozoa), which are more prevalent among those with poor personal hygiene habits. This indicates a need for an integrated approach to hygiene promotion, surveillance, and treatment.
Keywords
Coinfection
Human immunodeficiency virus and intestinal parasites
Helminths
Protozoa
Nasarawa-Nigeria
INTRODUCTION
Globally, there is still an upsurge in parasitic infections especially in Sub-Saharan Africa, to which Nigeria is not an exception. This increase is becoming predominantly more among HIV-infected groups; thus, several regions around the globe and populaces across Nigeria (in particular Nasarawa state) characterize enduring public health challenges and undermine gains made by health institutions in the State. Recent assessments show that one-quarter or even more are recurrently infected with intestinal parasites globally, among these numbers most are people living with human immunodeficiency (HIV) and acquired immunodeficiency syndrome (AIDS) especially in developing countries with huge economic challenges.[1-3] Most-at-risk groups (that is, HIV/AIDS) are vulnerable by various diseases, especially those triggered by diverse biological agents (such as intestinal parasites) which includes: Isospora belli, Entamoeba histolytica, Giardia intestinalis, Trichuris trichiura, Necator americanus, Cryptosporidium spp., Microsporidia spp., Strongyloides stercoralis, and Ascaris lumbricoides.[4] The opportunistic intestinal parasites’ are the major problems in such group of patients.[5] An estimated number of about 3.5 billion people are affected worldwide, while 450 million have suffered illnesses due to parasitic infections; major causes are as a result of poor personal cleanliness practice and sanitation.[5,6] Gastrointestinal parasites infections result in iron deficiency, anemia, immune suppression, growth retardation in children, and other physical and mental health problems.[5] Intestinal parasites infections are reportedly more prevalent among the poor segments of the human population.[7] They are common in developing countries like Nigeria and are closely associated with low household income, overcrowding, poor environmental sanitation and personal hygiene, lack of access to clean water, poor or complete absence of basic sanitary facilities, and favorable climate with low altitudes.[8]
Recently, the HIV prevalence rate stands at 1.4% in Nigeria and the North Central part or zone of Nigeria account for 2.1% prevalence rate with Nasarawa state having the second highest prevalence rate of 2.0% after Benue state which account for about 5.3%, and also, the survey showed that HIV prevalence rate among adults age 15–64 years in the zone is 2.1%.[9,10] The Nasarawa state SURGE 2019 project by institute of human virology, Nigeria showed that an estimated 23,306 people living with HIV require viral suppression.[11] The epidemic coupled with the COVID-19 outbreak has impacted all segments of the society, markedly eroding gains the government has made in some vital health indices and further reducing the life expectancy in Nigeria. It has further weakening and threatening to overwhelm the Nigerian health-care system, increased the number of orphans and vulnerable children,[12] and also increased costs of achieving set sustainable development goals by decreases the size of the workforce as it affects mainly adults in their most productive years of life (15–49 years).
MATERIAL AND METHODS
Study design and framework
Case–control study design was used for the study. Samples were collected from patients attending ARV clinics and attending other services (HIV-negative patients) from the study health facilities (that is, comprehensive health facilities) by a systematic random sampling approach. The study was conducted from October 11, 2021, to February 7, 2022. A total of 422 subjects consisting of 212 HIV-positive patients attending HIV clinics and 210 HIV-negative individuals were enrolled for the study. The age of the study subjects ranges from 18 to 70 years. A pre-designed structural questionnaire was utilized to collect the demographic, socioeconomic characteristics, household hygiene, and clinic results of the subjects. Blood and stool samples were collected from recruited participants that were attending ART clinic in selected facilities and participants that were HIV-negative at point of recruitment within the study areas. The study ensured that participation was voluntary and confidential.[13]
Description of the study area
Nasarawa is a state in the north central geopolitical zone in Nigeria and its capital is Lafia with its coordinates given as 09°32’N, 8°18’E occupying a landmass of about 27,117 km2 and a total population of 2,523,395 in 2016.[14,15] The study areas comprise four semi-urban areas representing four respective local government areas (LGAs) with high HIV prevalence rate in the state, namely, Sabon Gida in Kokona LGA, Panda in Karu LGA, Kwandere in Lafia LGA, and Nasarawa Eggon in Nasarawa Eggon LGA [Figure 1].
Sample selection and collection
Blood and stool specimens were collected from each subject. The blood specimens were placed in ethylenediaminetetraacetic acid containers and the stool specimens were collected in clean wide-mouthed containers. Stool specimens were examined microscopically for ova, cysts, or parasites, using saline and iodine mounts on grease-free slides. Following this, each fresh stool sample was preserved in 10% formol saline. The fecal sample preserved in 10% formol saline was concentrated and used to detect oocysts of Cryptosporidium species using the modified Ziehl–Neelsen staining technique.
Formol ether concentration technique
Approximately 1 g of fecal sample was taken from the collected sample 3 mL of 10% formol water emulsify the 1-g fecal sample, using an applicator stick or a rod. Additional 3 mL of 10% v/v formalin was added and mixed well by shaking. After shaking, the emulsified feces were sieved and collected in a beaker into which the filtered suspension was transferred.
Modified Ziehl–Neelsen method
This method was further used to diagnose for human intestinal Cryptosporidium parvum. A smear was prepared from the sediment obtained by the formol ether concentration technique. The smear was allowed to air-dry and fixed with methanol for 2–3 min. After fixing, it was stained with carbolfuchsin for 15 min and washed using distilled water. The smear was decolorized with 1% acid alcohol for 10–15 s and then washed with distilled water, after which it was counter stained with 0.5% methylene blue for 30 s. The slide was washed off with distilled water and allowed to air dry. It was examined microscopically for oocysts, using oil immersion objective (100×) to identify C. parvum (oocysts).
Screening of participants to determine HIV status
The HIV clients attending ART clinics were not screened as their HIV status was already known and were accessing drugs in the facilities. While, the 210 HIV-negative persons who participated in the research had blood samples drawn from them and were screened for HIV using the parallel algorithm test recommended by the Nigerian government through the National Agency for the Control of AIDS by testing them with Determine test kit and anyone with the positive result was re-tested with a unigold test kit and if positive they were disqualified to serve as control but when negative stat-Pak was used for a third diagnosis for final confirmatory test (which serve as the tie breaker).
- Local government areas where the four selected comprehensive health facilities are located.
Statistical analysis
The IBM SPSS statistical software, version 25.0 was used to set up the database for the data collected during the fieldwork and also used for analyzing the data. Categorical variables were analyzed using frequency and percentages for the demographic and socioeconomic information. A univariate statistical analysis was carried-out utilizing Pearson’s Chi-square test to compare the client’s HIV status and intestinal parasitic infection, and also, the test is used to determine the association between intestinal parasitic infection and the participants’ demographic and socioeconomic characteristics. Binary logistic regression technique was used to analyze the relationship of behavioral, sociodemographic factors, and immune variables with parasite-infection status (that is, infected = 1 and not infected = 0). A statistical test is reported significant if the test statistic is P < 0.05.
RESULTS
Study cohorts
In this study, 422 persons were recruited, which includes 212 HIV-positives and 210 HIV-negative serving as control. Blood samples were collected from 420 individuals and 411 participants submitted their stool samples while, only 412 participants answered the issued questionnaires. A total number of 411 participants completed the three phase s of the study framework, that is, complete data were obtainable from 411 participants who provided blood samples, stool samples, and filled-in the questionnaires, as shown in [Figure 2]. In the study, HIV-negative individuals identified with intestinal parasite s are used as controls and were used to match HIV-positive individuals with parasitic infection. It was identified that most parasitic cases recorded among the HIV-positive group are individuals who were newly tested with HIV infection.
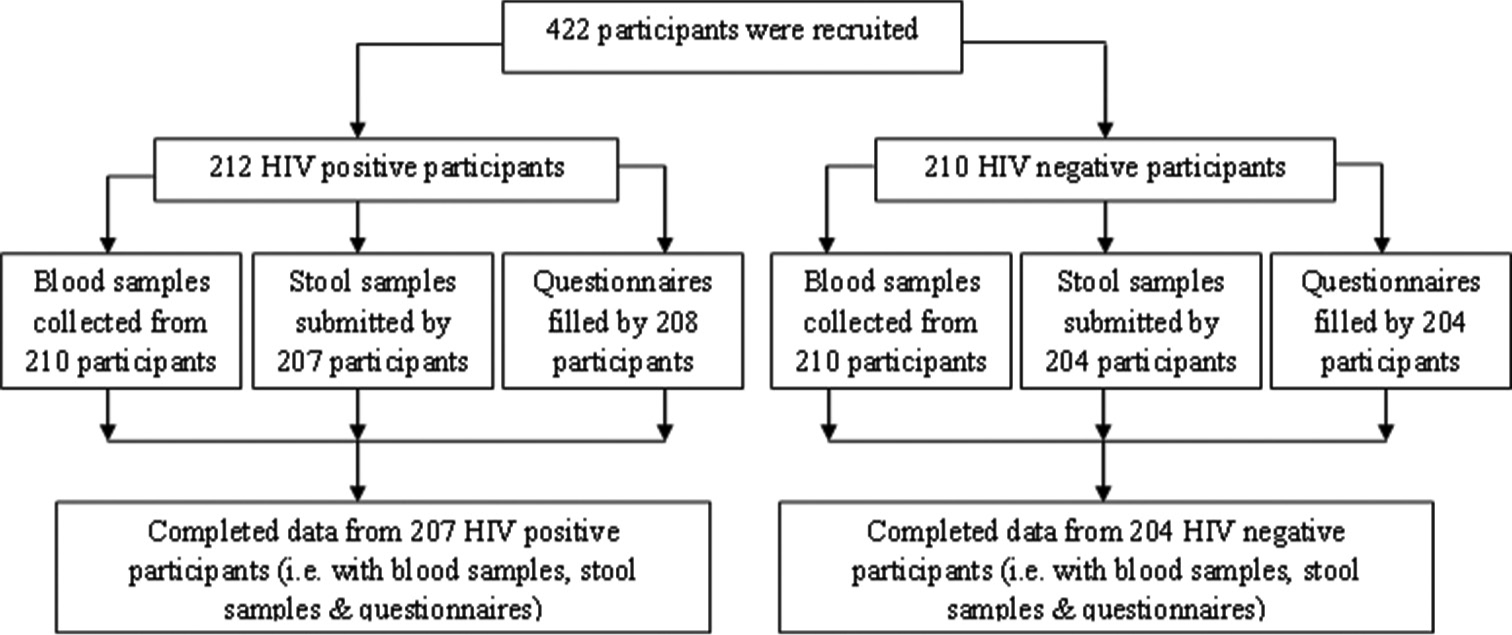
- Study participation on the coinfection between of human immunodeficiency virus-positive and negative individuals and intestinal parasitic infection.
Among the 411 participants who submitted stool samples 207 were HIV-positive and 204 HIV-negative. HIV-positives males comprised 104 (50.2%) and female s 103 (49.8%) with their mean (±SD) age given as 43.4 (±2.1) years. Among the HIV-negative individuals, both males and females comprise 102 (50.0%) each with their mean (±SD) age given as 42.1 (±1.9) years. The ratio of HIV-positive (male to female) was 0.50:0.49 and 0.50:0.50 in HIV-negative (male to female). The highest number of individuals examined for HIV-positive group are 59 (28.5%) within the range 42–49 years and 53 (26.0%) for HIV-negative group are within the range 34–41 years. The result showed that parasitic infections are higher among single individuals than any other groups under the marital status cohort with about 35% and 51% for HIV-positive and HIV-negative individuals [Table 1].
| Variable | HIV-positive | HIV-negative | χ2 (P-value) | |||
|---|---|---|---|---|---|---|
| HIV-positive (n=207) n (%) |
n (%) with parasite | HIV-negative (n=204) n (%) |
n (%) with parasite | |||
| Sex | 13.872 (0.001*) | |||||
| Male | 104 (50.2) | 29 (27.9) | 102 (50.0) | 25 (24.5) | ||
| Female | 103 (49.8) | 49 (47.6) | 102 (50.0) | 32 (31.4) | ||
| Average age (year)* | 43.4 (42.6, 40.9) | 42.1 (41.6, 40.1) | ||||
| Age | 0.176 (0.191) | |||||
| 18–25 | 28 (13.5) | 14 (50.0) | 29 (14.2) | 10 (34.5) | ||
| 26–33 | 33 (15.9) | 14 (42.4) | 47 (23.0) | 8 (17.0) | ||
| 34–41 | 41 (19.8) | 17 (41.5) | 53 (26.0) | 13 (24.5) | ||
| 42–49 | 59 (28.5) | 17 (28.8) | 51 (25.0) | 14 (27.5) | ||
| 50–57 | 21 (10.1) | 9 (42.9) | 13 (6.4) | 8 (61.5) | ||
| 58–65 | 19 (9.2) | 7 (36.8) | 9 (4.4) | 3 (33.3) | ||
| 66+ | 6 (2.9) | - | 2 (1.0) | 1 (50.0) | ||
| Marital status | 0.233 (0.533) | |||||
| Single | 74 (35.7) | 13 (17.6) | 104 (51.0) | 29 (27.9) | ||
| Married | 35 (16.9) | 26 (74.3) | 83 (40.7) | 24 (28.9) | ||
| Separated | 57 (27.5) | 21 (36.8) | 12 (5.9) | 4 (33.3) | ||
| Divorced | 41 (19.8) | 18 (43.9) | 5 (2.5) | - | ||
| Source of water | 9.877 (0.020*) | |||||
| Stream/river | 32 (15.5) | 26 (81.3) | 23 (11.3) | 21 (91.3) | ||
| Well | 55 (26.6) | 23 (41.8) | 49 (24.0) | 15 (30.6) | ||
| Street taps | 22 (10.6) | 9 (40.9) | 15 (7.4) | 7 (46.7) | ||
| In-house tap | 19 (9.2) | 4 (21.1) | 32 (15.7) | 3 (9.4) | ||
| Tankers | 13 (6.3) | 7 (53.8) | 10 (4.9) | 6 (60.0) | ||
| Bore hole | 15 (7.2) | 1 (6.7) | 24 (11.8) | - | ||
| Rain water | 13 (6.3) | 2 (15.4) | 8 (3.9) | 2 (25.0) | ||
| Water vendors | 38 (18.4) | 9 (23.7) | 43 (21.1) | 3 (7.0) | ||
| Method of Sewage | 0.766 (0.009*) | |||||
| Bush/field/river | 39 (18.8) | 25 (64.1) | 31 (15.2) | 15 (48.4) | ||
| Pit toilet | 50 (24.2) | 15 (30.0) | 57 (27.9) | 11 (19.3) | ||
| Ventilated IPL | 54 (26.1) | 11 (20.4) | 59 (28.9) | 9 (15.3) | ||
| Bucket | 24 (11.6) | 17 (70.8) | 18 (8.8) | 12 (66.7) | ||
| Water closet | 29 (14.0) | 3 (10.3) | 34 (16.7) | 5 (14.7) | ||
| Others | 11 (5.3) | 7 (63.6) | 5 (2.5) | 5 (100.0) | ||
| Educational status | 15.218 (<0.001)* | |||||
| No formal edu. | 57 (27.5) | 24 (42.1) | 59 (28.9) | 18 (30.5) | ||
| Qur’anic | 44 (21.3) | 19 (43.2) | 29 (14.2) | 9 (31.0) | ||
| Primary | 55 (26.6) | 21 (38.2) | 41 (20.1) | 15 (36.6) | ||
| Secondary | 33 (15.9) | 11 (33.3) | 63 (30.9) | 11 (17.5) | ||
| Tertiary | 18 (8.7) | 3 (16.7) | 12 (5.9) | 4 (33.3) | ||
| Occupation | 0.121 (0.722) | |||||
| Student | 9 (4.3) | 7 (77.8) | 19 (9.3) | 10 (52.6) | ||
| Pensioner | 17 (8.2) | 9 (52.9) | 16 (7.8) | 11 (68.8) | ||
| Business people | 55 (26.6) | 24 (43.6) | 57 (27.9) | 10 (17.5) | ||
| Farmers | 69 (33.3) | 26 (37.7) | 77 (37.7) | 17 (22.1) | ||
| Civil servant | 35 (16.9) | 9 (25.7) | 33 (16.2) | 9 (27.3) | ||
| Others | 22 (10.6) | 3 (13.6) | 2 (1.0) | - | ||
*Denotes significance at 0.05 level of significance. P value from Chi-square test. HIV: Human immunodeficiency virus, IPL: Improved pit latrine
Among the study subjects, 26.6% of HIV-positive individuals and 24.0% of HIV-negative use well water also, the study showed that about 18.4% and 21.1% representing HIV-positive and negative individuals, respectively, get their water sources from water vendors, as shown in Table 1. About 26.1% HIV-positive individuals use ventilated improved pit latrine (IPL). Among HIV-negative group 28.9% also use ventilated IPL as their sewage method. About 27.5% of HIV-positive individuals have no formal education which is the highest in among the groups. Among HIV-negative individuals, majority of the study subjects only have a secondary level of education (that is, 30.9%). Furthermore, the results showed that majority of the study subjects were farmers across the study areas. Among HIV-positive individuals, 69 (33.3%) are farmers, and 77 (37.7%) are farmers among HIV-negative individuals.
Intestinal parasitic infections
The overall prevalence of intestinal infections among HIV-positive individuals was 37.7% (78/207) and 27.9% (57/204) among HIV-negative individuals. The overall prevalence of intestinal geohelminth infections was 20.2% (83/411). The overall prevalence rate of geohelminth parasitic infections among HIV-positive individuals was 19.8% (41/207) and 20.6% (42/204) among HIV-negative individuals. While, the overall prevalence of intestinal Protozoa infections was 12.7% (52/411). The overall prevalence rate of Protozoa parasitic infections among HIV-positive individuals was 17.9% (37/207) and 7.4% (15/204) among HIV-negative individuals. The most common geohelminth parasite infections as identified by this study was hookworm with 7.1% (29/411), followed by Tape worm with a rate of 6.3% (26/411). As shown in [Table 2], individuals that are HIV-positive were more infected by hookworm, A. lumbricoides, and Tape-worm at a rate of 9.7% (20/207), 5.3% (11/207), and 3.4% (7/207), respectively, with a significant difference between infections statuses with each of these intestinal geohelminths and HIV serotype since their respective P-values are significant (that is, P < 0.05, Table 2). Giardia lamblia was mostly identified (5.6%; 23/411) among the Protozoa parasites followed by E. histolytica with a rate of 4.6% (19/411). HIV-positive groups were identified with G. lamblia, E. histolytica, Cryptosporidium spp., Escherichia coli, and Entamoeba hartmanii at a rate of 8.2% (17/207), 6.8% (14/207), 1.5% (3/207), 1.0% (2/207), and 0.5% (1/207), respectively. The result, as shown in [Table 2], indicates that significant difference between rates among HIV serotype was only found in the case of G. lamblia and E. histolytica infections which were the most common parasitic infections among HIV-positives (P < 0.05; Table 2).
| Parasites | HIV-positive (n=207) number (%) | HIV-negative (n=204) number (%) | χ2 (P-value) |
|---|---|---|---|
| Geohelminths | |||
| Ascaris lumbricoides | 11 (5.3) | 8 (3.9) | 10.219 (0.002*) |
| Trichuris trichiura | 3 (1.5) | 6 (2.9) | 1.199 (0.432) |
| Hookworm | 20 (9.7) | 9 (4.4) | 8.323 (0.007*) |
| Tape worm | 7 (3.4) | 19 (9.3) | 0.436 (0.032*) |
| Protozoa | |||
| Entamoeba histolytica | 14 (6.8) | 5 (2.5) | 9.087 (0.002*) |
| Entamoeba hartmanii | 1 (0.5) | 1 (0.5) | 0.161 (0.764) |
| Escherichia coli | 2 (1.0) | 1 (0.5) | 0.143 (0.911) |
| Giardia lamblia | 17 (8.2) | 6 (2.9) | 7.321 (0.007*) |
| Cryptosporidium spp. | 3 (1.5) | 2 (1.0) | 0.199 (0.712) |
*Denotes significance at 0.05 level of significance. P-value from Chi-square test. HIV: Human immunodeficiency virus
Community/LGAs parasitic infection rate
Intestinal parasitic infection is more prevalent in the Sabon gida community representing Kokona LGA with a rate of 10.7% (44/411) followed by the Nasarawa Eggon community (Nasarawa Eggon LGA with a rate of 9.7% (40/411), while Panda (Karu) and Kwandere (Lafia LGA) have the lowest rates of 6.8% (28/411) and 5.6% (23/411), respectively [Table 3].
| Semi-urban settlements |
LGAs | Total enrolment |
Stool submission |
No. (%) HIV+ | No. (%) with parasite | No. (%) HIV− | No. (%) with parasite |
|---|---|---|---|---|---|---|---|
| Sabon gida | Kokona | 118 | 115 | 62 (53.9) | 28 (45.2) | 53 (46.1) | 16 (30.2) |
| Panda | Karu | 101 | 98 | 54 (55.1) | 17 (31.5) | 44 (44.9) | 11 (25.0) |
| Kwandere | Lafia | 94 | 91 | 48 (52.7) | 12 (25) | 43 (47.3) | 11 (25.6) |
| Nasarawa Eggon | Nasarawa Eggon | 109 | 107 | 43 (40.1) | 21 (48.8) | 64 (59.8) | 19 (29.7) |
| Total | 422 | 411 | 207 (50.4) | 78 (37.7) | 204 (49.6) | 57 (27.9) |
Total number of parasitic infection: 135, HIV-positive with parasites: 78 and HIV-negative with parasites: 57. LGAs: Local government areas, HIV: Human immunodeficiency virus
Among HIV-positive individuals, the result also indicates that Sabon Gida (Kokona LGA) has more confirmed parasitic cases (13.5%; 28/207) which is followed by Nasarawa Eggon (Nasarawa Eggon LGA), Panda (Karu LGA), and Kwandere (Lafia LGA) at a rate of 10.1% (21/207), 8.2% (17/207), and 5.8% (12/207), respectively. While, for HIV-negative individuals, Nasarawa Eggon (Nasarawa Eggon LGA) has the highest rate of about 9.3% (19/204) followed by Sabon Gida (Kokona LGA) with 7.8% (16/204) and 5.4% (11/204) for both Panda (Karu LGA) and Kwandere (Lafia LGA) each.
Parasitic infection, demographic, and socioeconomic characteristics
The Chi-square P-values obtained in [Table 1] shows that sex, source of water, method of sewage, and educational status of participants with Chi-square test values (and corresponding P-values) given as 13.872 (0.001), 9.877 (0.020), 0.766 (0.009), and 15.218 (P < 0.001) have significant association with parasite infections among HIV-positive and negative individuals since their probability values are <5% level of significance (that is, P < 0.05). This result indicates that intestinal parasitic infections as recorded in this study across the study areas representing each LGAs largely depends on sex, source of water, method of sewage disposal, and the educational level of the study groups.
Risk factors for relationship between HIV and Hookworm (geohelminth) and G. lamblia (Protozoa)
The result indicates that among the 207 HIV-positive subjects that submitted their stool samples were identified more with hookworm and G. lamblia [Table 2]. The binary logistic regression analysis shows that sex (1.823; 0.002 < 0.05), water treatment (−1.459; 0.015 < 0.05), covering of water vessels (−1.848; 0.001 < 0.05), diarrhea (−0.952; 0.043 < 0.05), and good hygiene practice (−1.978; P < 0.001) were significantly associated with hookworm (Geohelminth) in the study areas [Table 4]. While, water treatment (−1.698; 0.035 < 0.05), good nutrition (−0.789; 0.048 < 0.05), covering of water vessels (1.765; 0.021 < 0.05), and good hygiene practice (0.898; 0.042) were significantly associated with G. lamblia (Protozoa). Furthermore, it can be seen that water treatment, covering water, and good hygiene have negative association with parasitic infection occurring the highest among the HIV-positive subjects [Table 4].
| Variables | Regression coefficient (B) | Standard error | P-value | OR | 95% CI (OR) |
|---|---|---|---|---|---|
| Risk factors for HIV and Hookworm | |||||
| Sexa | 1.823 | 0.653 | 0.002* | 3.191 | (2.113, 5.432) |
| Ageb <43 years | 1.652 | 0.712 | 0.077 | 2.192 | (0.082, 3.736) |
| Water treatmentb | −1.459 | 0.533 | 0.015* | 0.300 | (0.221, 0.490) |
| Good nutritionb | −0.761 | 0.675 | 0.062 | 0.141 | (0.091, 0.221) |
| Storage vessel (s) have a coverb | −1.848 | 0.644 | 0.001* | 0.348 | (0.171, 0.491) |
| Diarrhea in the last 6 monthsb | 0.952 | 0.599 | 0.043* | 1.521 | (0.112, 2.891) |
| Good hygiene practiceb | −1.978 | 0.581 | <0.001* | 0.229 | (0.173, 0.382) |
| Risk factors for HIV with G. lamblia | |||||
| Sexa | −0.742 | 0.448 | 0.066 | 0.476 | (0.121, 2.167) |
| Ageb <43 years | −0.654 | 0.356 | 0.087 | 0.520 | (0.189, 3.982) |
| Water treatmentb | −1.698 | 0.744 | 0.035* | 0.464 | (0.190, 1.198) |
| Good nutritionb | −0.789 | 0.343 | 0.047* | 0.654 | (0.186, 2.235) |
| Storage vessel (s) have a coverb | 1.765 | 0.245 | 0.021* | 0.844 | (0.200, 3.154) |
| Diarrhea in the last 6 monthsb | −0.568 | 0.678 | 0.099 | 1.767 | (0.111, 3.009) |
| Good hygiene practiceb | 0.898 | 0.436 | 0.042* | 0.492 | (0.109, 2.765) |
*Denotes significance at 0.05 level of significance of the regression coefficient. a(male=1; female=0), b(yes=1; no=0). HIV: Human immunodeficiency virus, G. lamblia: Giardia lamblia, OR: Odds ratio, CI: Confidence interval
The result showed that there is higher risk of hookworm infection among males than females (odds ratio [OR] = 3.191, confidence interval [CI]: 2.113, 5.432) and study subjects younger than 43 years (OR = 2.192, CI: 0.082, 3.736) as well as higher risk among subjects with diarrhea (OR = 1.521, CI: 0.112, 2.891, Table 4). While, study subjects were at lower risk of being infected with hookworm if they treat their water before usage (OR = 0.300, CI: 0.221, 0.490), a good nutritional status (OR = 0.141, CI: 0.091, 0.221), proper coverage of water vessels (OR = 0.348, CI: 0.171, 0.491), and good hygiene practice (OR = 0.229, CI: 0.173, 0.382). The binary logistic regression analysis [Table 4] showed lower risk of G. lamblia among males than females (OR = 0.476, CI: 0.121, 2.167) and study subjects younger than 43 years (OR = 0.520, CI: 0.189, 3.982). And also, lower risk of G. lamblia among study subjects that observed the following explanatory variables such as water treatment (OR = 0.464, CI: 0.190, 1.198), a good nutritional status (OR = 0.654, CI: 0.186, 2.235), proper coverage of water vessels (OR = 0.844, CI: 0.200, 3.154), and good hygiene practice (OR = 0.492, CI: 0.109, 2.765). While, G. lamblia prevalence was higher among study subjects with diarrhea (OR = 1.767, CI: 0.111, 3.009).
DISCUSSION
The study showed that the overall prevalence of intestinal parasitic infection in the study areas was 32.8% and high among HIV-positive individuals, at approximately 37.7%. The prevalence of geohelminths was 5.6%, with hookworm being the most common species identified. This result is in agreement with a study conducted in rural areas of China[5] and in Nigeria.[16] While, the prevalence of Protozoa was 9.0% with G. lamblia been the most common among the Protozoa species as identified by the study as in HIV-positive individuals.[5] Our findings showed that there is a higher risk of hookworm infection among males than females and study participants younger than 43 years as well as higher risk among participants with diarrhea and lower risk of being infected with hookworm if study participants treat their water before usage, a good nutritional status, proper coverage of water vessels, and conduct good hygiene practice. Regarding G. lamblia, the study deduced that there is a lower risk of infection among males than females and study subjects younger than 43 years as compared to hookworm prevalence. In addition, the study found that there is a lower risk of G. lamblia infection among study subjects who observed the following explanatory variables: water treatment, a good nutritional status, proper coverage of water vessels, and good hygiene practices. The result also showed that G. lamblia prevalence was higher among study subjects with diarrhea. This result is in agreement with a study conducted at Hawassa Teaching and Referral Hospital in Ethiopia.[17]
HIV infection would definitely increase risks of intestinal parasitic infection, especially geohelminth infection. Our study results supports this claim as intestinal parasitic infection was higher in HIV-positive individuals (that is, overall prevalence of about 37.7%) as compared to HIV-negatives (27.9%); this is due to the fact that their immune system is already comprised and majority of the HIV-positive clients in these locations live below the poverty line.[18,19] The study revealed that intestinal parasitic infection is more prevalent in the Sabon Gida community representing Kokona LGA followed by Nasarawa Eggon community (Nasarawa Eggon LGA). The result also indicates that among HIV-positive individuals, Sabon Gida (Kokona LGA) have more confirmed parasitic cases also followed by Nasarawa Eggon (Nasarawa Eggon LGA). While, among HIV-negative individuals, Nasarawa Eggon (Nasarawa Eggon LGA) has the highest intestinal parasitic cases followed by Sabon Gida (Kokona LGA); majority of the clients live in slums and have poor hygiene practice with poor access to treated water and sanitary systems.
The study also showed that sex, source of water, method of sewage, and educational status of participants have significant association with parasite infections among HIV-positive and negative individuals; this result is in agreement with a study conducted in selected areas of Eastern Cape.[20] This result indicates that intestinal parasitic infections as recorded in this study across the study areas representing each LGAs largely depends on sex, source of water, method of sewage disposal, and the educational level of the study groups. The study indicated that pit toilet is the most used sewage method in the selected study areas this is followed by ventilated IPL and bush/field/river among the study participants. Meanwhile, intestinal parasitic infection was more prevalent among study participants who use that use bush/field/river, pit toilet, and bucket as method of sewage. The study revealed that about 25.3% of the study subjects uses well water which constitutes about 9.2% intestinal infection rate among the entire study participants; study participants who get their water from water vendors constitute the second highest with about 19.7% with intestinal prevalence rate of 2.9%. Our findings showed that stream/river water is the third most commonly used water source, with 13.4% of the entire sample using it, resulting in an intestinal prevalence rate of approximately 11.4%.
The study evaluated some risk factors associated with the prevalence of hookworm and G. lamblia infection among HIV-positive individuals. From the findings, it can be deduced that water treatment, covering of water vessels, and good hygiene practice were identified in both parasitic infections and these variables have significantly associated with both intestinal parasites infections occurring the most among the HIV-positive subjects as a result of the compromised immune system. The binary logistic regression analysis showed that there is higher risk of hookworm infection among males than females these maybe as a result of low male attendance at health-care facilities to seek medical care in the study area; this result is in line with a study conducted at University of Gondar in Northwest Ethiopia.[21] The study revealed that hookworm infection is more prevalent in subjects younger than 43 years and higher risk among subjects with diarrhea. While, study subjects were at lower risk of being infected with hookworm if they treat their water before usage, a good nutritional status, proper coverage of water vessels, and practice good hygiene. Furthermore, the binary logistic regression analysis showed lower risk of G. lamblia among males than females and study subjects younger than 43 years. And also, lower risk of G. lamblia among study subjects that observed the following explanatory variables such as water treatment, a good nutritional status, proper coverage of water vessels, and good hygiene practice. While, G. lamblia prevalence was also higher among study subjects with diarrhea; this result is in line with this study.[22]
CONCLUSION
The upsurge in intestinal parasitic infection among the study participants requires urgent intervention that is strategically structured to avoid severe consequences especially among the HIV-positive clients. The study identified hookworm and G. lamblia, as significantly higher among the participants that are HIV-positive and particularly a higher risk of hookworm among males than females and study subjects younger than 43 years as well as higher risk among subjects with diarrhea, while, for G. lamblia, higher risk was found among subjects with diarrhea. There is need for targeted interventions geared toward personal hygiene habits, good nutrition, and water treatment programs as these may help reduce the high prevalence rate of intestinal parasite as identified by this study, especially the HIV-positive participants and also reduce the chances of diarrhea occurrence.
Ethical approval
The study was approved by the Nasarawa State Ministry of Health and supported by the Nasarawa State Agency for the Control of AIDS (NASACA). Participants were contacted through the hospital authorities and the leadership of the support group and the objectives, procedures, and potential risks carefully explained to all potential participants. Interested individuals were provided written informed consent in person before inclusion in the study. All potential participants were offered professional counseling before and after HIV testing by trained HTC Counselors, and all diagnostic results were kept strictly confidential. Free deworming drugs (albendazole and praziquantel) were offered to all participants found to be infected with the parasites through the health-care providers.
Acknowledgment
We are highly grateful to the study participants and acknowledge their time, effort, and collaboration during the field exercise of this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Prevalence of gastro-intestinal parasite in relation to availability of sanitary facilities among schooling children in Makurdi, Nigeria. Anim Res Int. 2006;3:489-93.
- [CrossRef] [Google Scholar]
- Evidence for a new species of Cryptosporidium infecting tortoises: Cryptosporidium ducismarci. Parasit Vectors. 2010;3:21-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention and Control of Intestinal Parasite Infections In: WHO Technical Report Series. Geneva: World Health Organization; 2002. p. :749.
- [Google Scholar]
- Prevalence of intestinal parasitic infections among HIV patients in Benin city, Nigeria. Libyan J Med. 2010;5:5506-71.
- [CrossRef] [PubMed] [Google Scholar]
- Co-infection of HIV and intestinal parasites in rural area of China. Parasit Vectors. 2012;5:36-2.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of intestinal parasitic infections among urban dwellers in Southwest Ethiopia. Ethiop J Health Dev. 2005;21:12-7.
- [CrossRef] [Google Scholar]
- Intestinal helminth parasites in school children in Iragbiji, Boripe local government, Osun state, Nigeria. Afr J Biomed Res. 2006;9:66-3.
- [CrossRef] [Google Scholar]
- Ending the AIDS Epidemic by 2030 United States: United State Agency for International Development; 2019.
- [Google Scholar]
- The Nasarawa State SURGE 2019 Project Nasarawa State, Nigeria: Institute of Human Virology; 2019.
- [Google Scholar]
- Modeling coinfection dynamics of HIV&AIDS, tuberculosis and hepatitis C virus. Sci World J. 2020;15:24-32.
- [Google Scholar]
- Neonatal pain relief and the Helsinki declaration. J Law Med Ethics. 2008;36:803-23, 611
- [CrossRef] [PubMed] [Google Scholar]
- Report of Nigeria's national population commission on the 2006 census. Popul Dev Rev. 2007;33:210-6.
- [Google Scholar]
- Demographic Statistics Bulletin 2017 Abuja: National Bureau of Statistics; 2017. p. :26-1.
- [Google Scholar]
- Gastrointestinal parasitic infections and immunological status of HIV/ AIDS coinfected individuals in Nigeria. Ann Glob Health. 2019;85:99.
- [CrossRef] [PubMed] [Google Scholar]
- Intestinal parasitic infections in relation to HIV/AIDS status, diarrhea and CD4 T-cell count. BMC Infect Dis. 2009;9:155.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of intestinal parasitic pathogens in HIV-seropositive individuals in Northern India. Jpn J Infect Dis. 2002;55:76-83.
- [Google Scholar]
- Intestinal parasitic infections in Thai HIV-infected patients with different immunity status. BMC Gastroenterol. 2001;1:3.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of intestinal parasites in HIV/AIDS-Infected patients attending clinics in selected areas of the Eastern Cape. Microbiol Res. 2022;13:583-74.
- [CrossRef] [Google Scholar]
- Intestinal parasites among HIV/AIDS patients attending university of Gondar hospital, Northwest Ethiopia. Ethiop J Health Dev. 2019;33:64-72.
- [Google Scholar]
- Prevalence of intestinal parasites in a cohort of HIVinfected patients from Antioquia, Colombia. Biomedica. 2021;41:153-64.
- [CrossRef] [PubMed] [Google Scholar]






