Translate this page into:
Scleroderma dermatomyositis overlap syndrome
*Corresponding author: Meenakshi Kalyan, Department of General Medicine, Vydehi Institute of Medical Science and Research Centre, Bengaluru, Karnataka, India. drmeenakshi.kalyan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Arora S, Kalyan M, Kolli C, Kumar D. Scleroderma dermatomyositis overlap syndrome. Sri Ramachandra J Health Sci. 2024;4:31-4. doi: 10.25259/SRJHS_49_2023
Abstract
A young female presented with oligoarthritis of the right hand, painful symmetrical quadriparesis, and darkening skin for 7 months. General physical examination showed hyperpigmentation on the face, neck, upper extremities, calcinosis cutis with swelling and tenderness in the right wrist, and interphalangeal joints with restriction of movements. Neurological examination revealed power in both upper limbs of 3/5 and 4/5 in both lower limbs, all deep tendon reflexes were diminished, and bilateral plantar was flexors. Investigations revealed creatinine phosphokinase – 3991 U/L, lactate dehydrogenase – 1009 mg/dL, antinuclear antibodies by enzyme-linked immunosorbent assay showed anticentromere antibodies positive, and anti-Mi2 positive. Electromyography was suggestive of myopathy. A muscle biopsy confirmed the diagnosis of dermatomyositis (DM). Hence, the diagnosis of limited scleroderma DM overlap syndrome was made. Serial electrocardiography showed sinus bradycardia and ventricular premature ectopics followed by sinus rhythm. She was treated with pulse therapy steroids, hydroxychloroquine, sulfasalazine, antiplatelets, and isoprenaline for cardiac involvement, with improvement in her symptoms.
Keywords
Myositis
Anticentromere antibodies
Anti-Mi2 antibodies
Muscle biopsy
INTRODUCTION
Overlap syndrome is defined as the classification criteria of at least two connective tissue diseases (CTDs) occurring at the same or at different times in the same patient, which includes systemic lupus erythematosus, rheumatoid arthritis (RA), scleroderma or systemic sclerosis (SSc), polymyositis/dermatomyositis (PM/DM), and Sjögren syndrome.[1] The prevalence of myositis in scleroderma 5.9%.[2] In the course of SSc, 20–30% of patients develop overlap syndrome.[3] It is important to identify scleroderma myositis overlap for close monitoring and early treatment as there is an increased prevalence of cardiac involvement and poor survival[4] in such patients.
CASE REPORT
A 26-year-old married female with two children presented with pain in the right wrist, interphalangeal joints associated with swelling and morning stiffness for more than 1 h, and painful symmetrical proximal weakness in all four limbs for 7 months. There was no history of distal muscle weakness in all four limbs, neck muscle weakness, dysphagia, respiratory distress, or symptoms of any cranial nerve involvement. There was no fever, seizures or, altered sensorium, speech abnormalities or, bladder/bowel disturbances, any root pains, thinning, stiffness of limb, or any sensory involvement. She had darkening of skin on the face, neck, and upper extremities for 7 months, not associated with photosensitivity, and no discoloration of hands-on exposure to cold or any digital/oral ulcers. There was no past or family history. General physical examination showed body mass index – 19.5, pulse (p), –78/min, regular, all peripheral pulses well felt, blood pressure – 110/70 mmHg, and respiratory rate (RR) – 14/min. Hyperpigmentation seen on the face, neck, upper extremities [Figure 1], calcinosis cutis, swelling, and tenderness in the right wrist and interphalangeal joints [Figure 2] with restriction of movements and no redness. Nails, hair, oral cavity, and thyroid were normal. There was no pallor, icterus, clubbing, cyanosis, lymphadenopathy, neurocutaneous markers, or thickened nerves. The neurological system showed higher mental functions, cranial nerves and bulk of muscles were normal, and hypotonia in all four limbs. Power in both upper limbs was 3/5 and 4/5 in both lower limbs, all deep tendon reflexes were diminished, and bilateral plantar were flexors. Sensory system was normal. The rest of the systemic examination was unremarkable. Investigations showed hemoglobin – 10.8 g/dL, white blood cell – 8400/cumm, platelets – 456,000, erythrocyte sedimentation rate of 92 mm/h, RA factor positive, total bilirubin – 1.0 mg/dL, direct bilirubin – 0.7 mg/dL, alanine aminotransferase – 242, aspartate aminotransferase – 92, alkaline phosphatase – 90 mg/dL, and hemoglobin A1c – 4.9%. Renal function test, serum electrolytes, thyroid function test, urine examination, chest X-ray, ultrasound abdomen, computed tomography- chest, and upper gastrointestinal endoscopy was normal. 24 h urine for proteins – 0.29 mg/day, creatinine phosphokinase (CPK) – 3991 U/L, and lactate dehydrogenase – 1009 mg/dL. lipids profile showed total cholesterol – 232 mg/dL, triglycerides – 504 mg/dL, high-density lipoprotein – 40 mg/dL, and low-density lipoprotein – 146 mg/dL. Electrocardiography (ECG) on initial presentation was normal. On day 6, following mild chest discomfort, ECG showed sinus bradycardia [Figure 3], Cardiac enzymes revealed creatine phosphokinase MB – 179 mg/dL and troponin I – 0.03 mg/dL. Echocardiography showed mild mitral regurgitation, bradycardia noted during study, mild tricuspid regurgitation, right ventricular systolic pressure – 26 mmHg, ejection fraction – 60%, and inferior vena cava < 50% collapsing. Subsequent ECG showed ventricular premature ectopics [Figure 4] followed by sinus rhythm [Figure 5]. Holter monitoring showed ventricular premature ectopics. Antinuclear antibodies (ANAs) by enzyme-linked immunosorbent assay showed anticentromere antibodies positive, anti-Mi2 positive, and U1RNP antibodies were negative. Electromyography was suggestive of myopathy. Muscle biopsy with H&E paraffin sections [Figure 6] and H&E cryosections [Figure 7] showed partially effaced fascicular architecture and muscle fibers polygonal to rounded with moderate variation in size. Florid myonecrosis, myophagocytosis, and regenerating fibers were seen with perivascular and interstitial lymphocytic inflammation in the endo and perimysium. Perifascicular atrophy was seen. Based on these findings, a diagnosis of scleroderma-DM overlap syndrome was made. She was initiated with I.V Methylprednisolone for 5 days, T. HCQ 200 mg BD, T. Sulfasalazine 500 mg BD, and Atorvastatin + Fenofibrate (145/10) OD. On day 6 of her admission, following the chest discomfort, elevated cardiac enzymes, and bradycardia, she was started with aspirin + clopidogrel + statin, i.v. atropine 2 mg, and low molecular weight heparin s.c for 5 days following which ECG reverted to heart rate of 100/min and in sinus rhythm. Subsequently initiated with T. Orciprenaline 10 mg total dissolved solids and oral prednisolone tapering dose. On follow–up, power in all 4 limbs was 4/5, and repeat CPK levels were 1831 U/L.

- Hyperpigmentation seen on the face and neck.
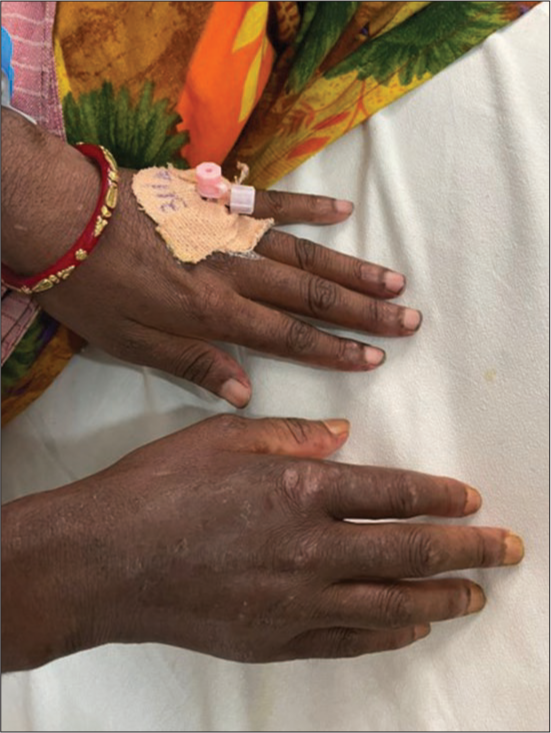
- Calcinosis cutis, swelling, and tenderness in the right wrist, interphalangeal joints.
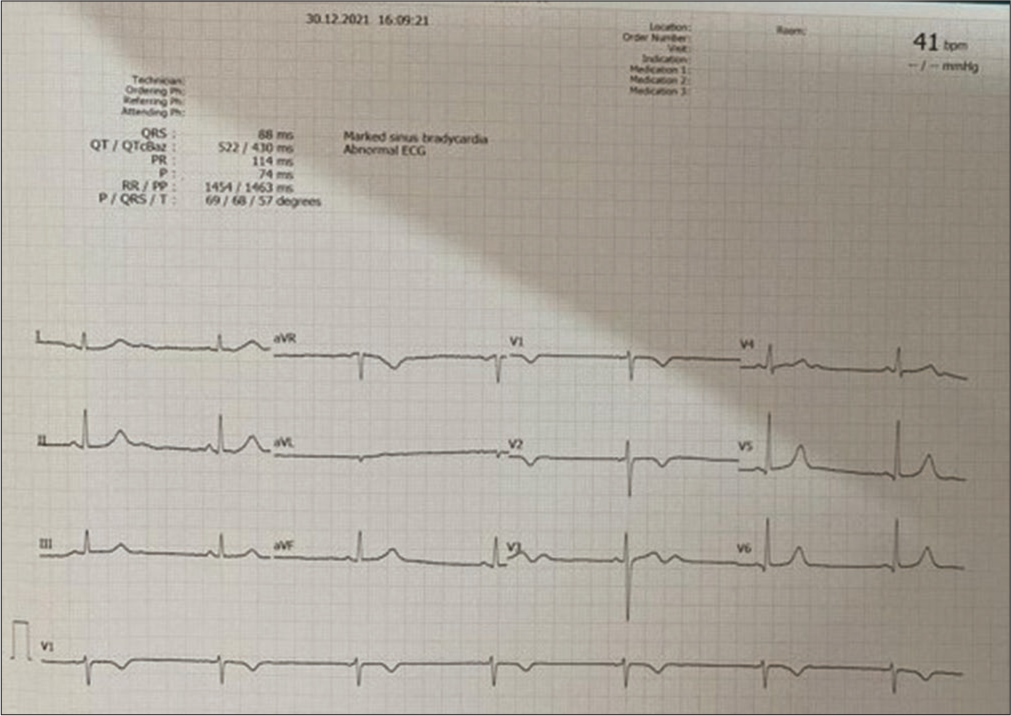
- Electrocardiography showed sinus bradycardia.
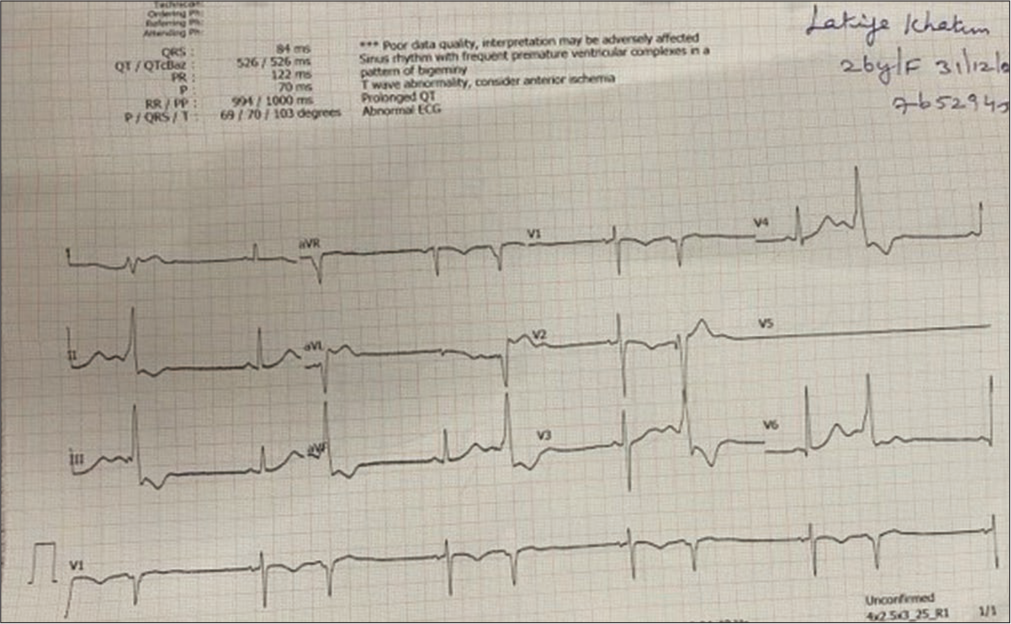
- Electrocardiography showed ventricular premature ectopics.
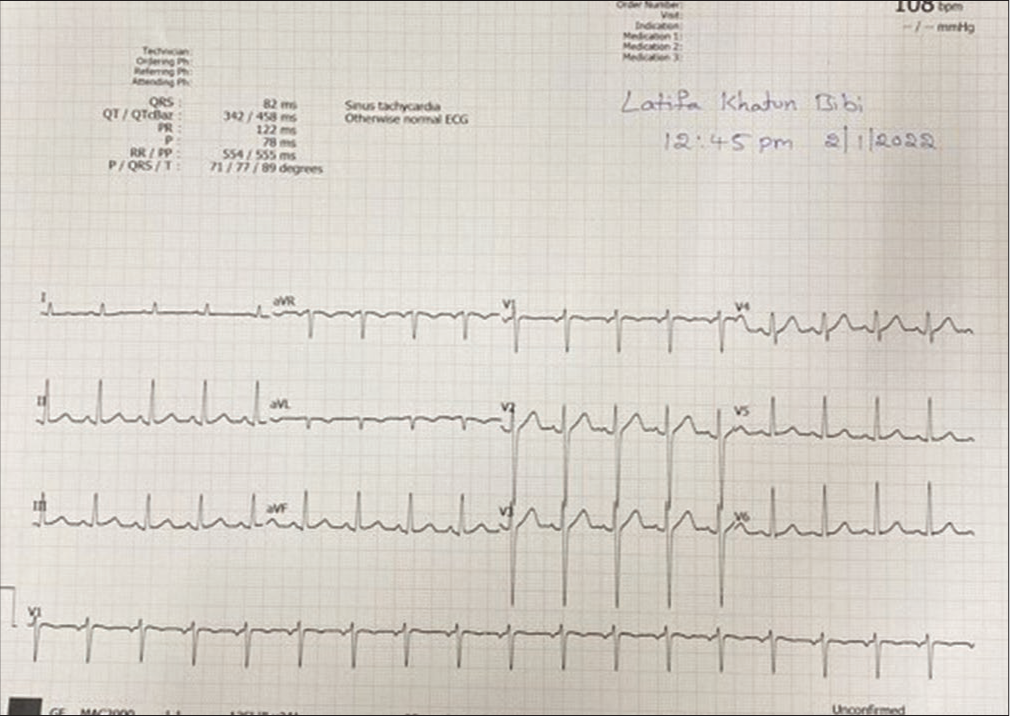
- Electrocardiography showing sinus rhythm.
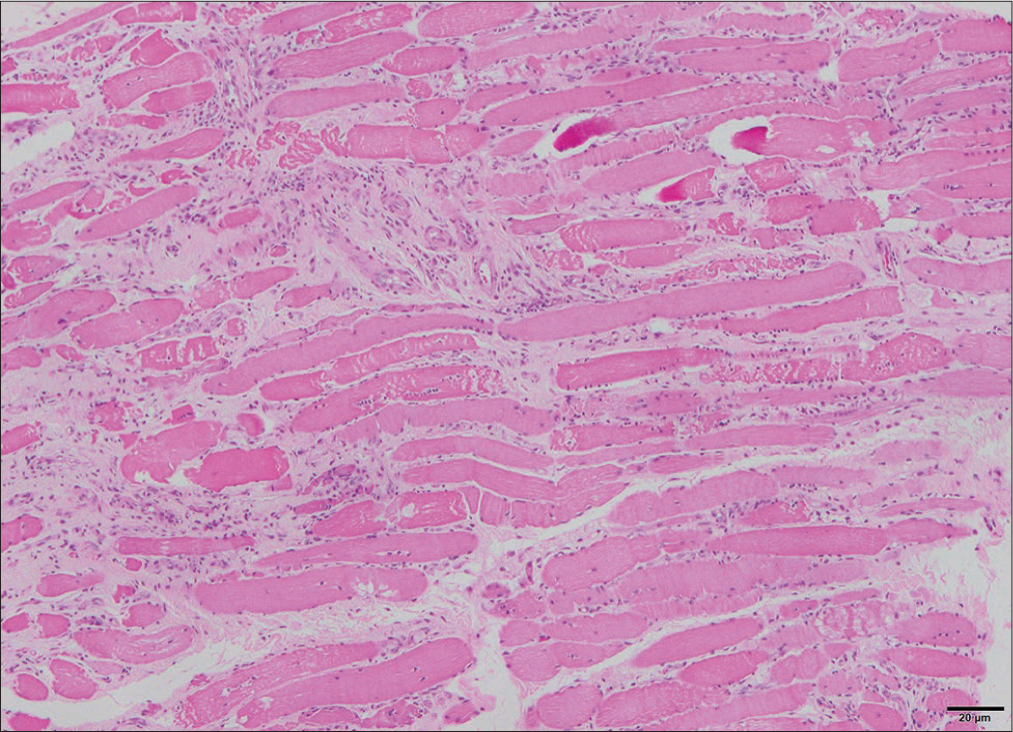
- Muscle biopsy with H&E paraffin sections. H&E: Haematoxylin and eosin stain.
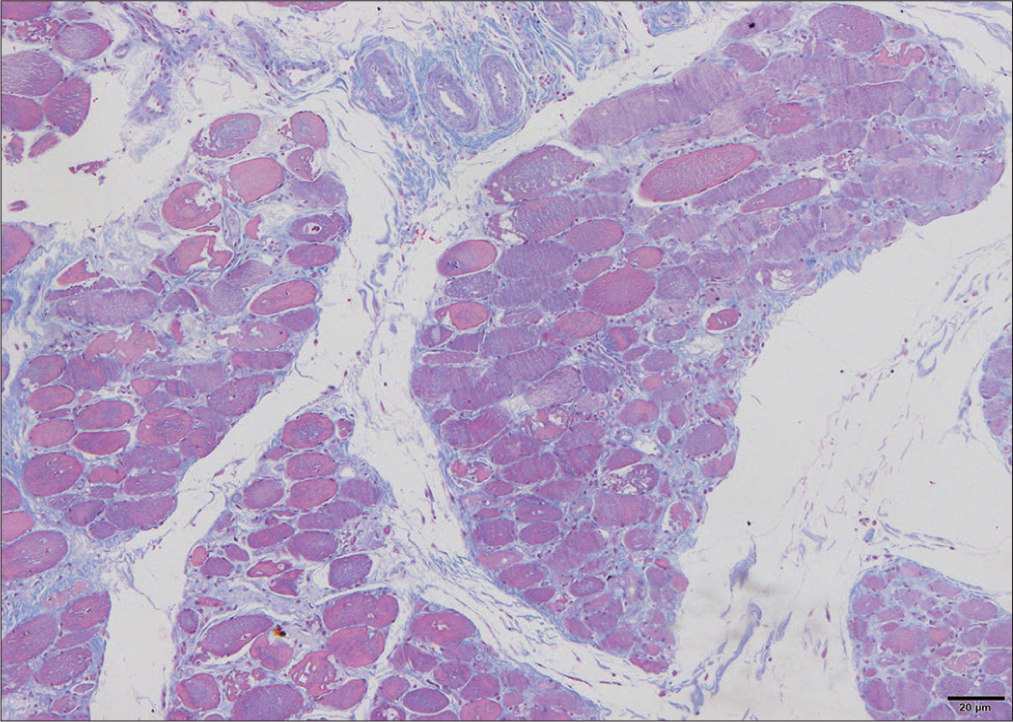
- Muscle biopsy with H&E cryosections. H&E: Haematoxylin and eosin stain.
DISCUSSION
Calcinosis cutis is the deposition of insoluble calcium in the skin and subcutaneous tissue. Autoimmune CTD-associated calcinosis is of a dystrophic type which occurs at sites of damaged tissue in the setting of normal serum calcium and phosphate levels manifesting most frequently in SSc and juvenile DM followed by adult DM The prevalence of Raynaud’s phenomena (RP) in systemic sclerosis (SSc) has been as high as 98%.[5] There may be 0.2% of patients with SSc, without RP or circulating ANA.[6] Our patient had no history of RP, but anticentromere antibodies were positive. Calcinosis in SSc occurs most frequently in the hands, fingers whereas in DM, it affects mainly the trunk, extremities, and pressure sites.[7] Calcinosis in DM and SSc shares some pathogenic mechanisms but vascular hypoxia plays an important role in systemic sclerosis. Autoantibodies often serve as markers of distinct clinical phenotypes in DM and SSc and as such several have been associated with calcinosis.[8] Antibodies are frequently reported in patients with overlap syndromes of myositis and systemic sclerosis.[9] Conventionally, calcinosis in systemic sclerosis has been linked to positive anti centromere antibody. The fact that the index finger and thumb are the two digits most affected by SSc-related calcinosis which was found in our patient. Polydermatomyositis and scleroderma overlap syndromes are now classified as distinct inflammatory myopathies entities; therefore, the new classification takes into account serological profiles. The risk of cardiac involvement is elevated in patients with SSc with rapidly progressing skin disease and concomitant skeletal myopathies. The pathogenesis of SSc cardiac involvement involves recurrent coronary microvascular ischemia and myocardial inflammation, leading to ischemic necrosis, reperfusion damage, and myocardial fibrosis. Acute or subacute myocarditis may also occur and, when severe, can be associated with skeletal muscle myositis,[10] which may represent a high-risk disease subset with an SSc and myositis overlap syndrome. Up to 42% of myositis patients with overlap CTD have been reported to have SSc.[11] Our patient had hyperpigmentation of skin on sun-exposed parts and no evidence of heliotrope rash or Gottron papules, but muscle biopsy was compatible with DM. DM specific antibodies are anti-Mi2 antibodies are positive in this patient with its clinical association of classic skin and muscle disease with good prognosis. Cardiovascular diseases such as valvular disease, arrhythmias, myocarditis, pericarditis, pulmonary hypertension, and coronary heart disease are frequent in PM/DM but typically remain subclinical. Timely and accurate diagnosis of cardiac involvement is warranted, but recognition of this disease entity is often delayed in clinical practice. Early detection of cardiac manifestations provides the opportunity for the early therapeutic intervention, which would improve the outcome. SSc is a clinical diagnosis, and skin biopsy or deep tissue biopsy should be performed if scleroderma-like disorder (e.g., eosinophilic fasciitis and scleromyxedema) is suspected and need to confirm the diagnosis.[12] Calcinosis is a late complication in patients with SSc, presenting many years after the first non-Raynaud symptom.[13] The association between calcinosis and long-duration disease in SSc and DM suggests that cumulative disease damage may play a role in the development of calcinosis, resulting in pain, ulcerations, infections, and impaired quality of life. Calcinosis is a significant clinical problem for patients with autoimmune CTD, especially SSc and DM, but they differ in distribution, composition and pathogenic mechanisms. Early aggressive treatment of underlying myositis can improve the long-term outcomes of calcinosis. A better understanding of the pathophysiology of calcinosis is needed to improve treatment options.
CONCLUSION
Scleroderma overlap syndromes should be regarded as a separate subset due to different progression of disease.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747-55.
- [CrossRef] [PubMed] [Google Scholar]
- Scleroderma-polymyositis overlap syndrome versus idiopathic polymyositis and systemic sclerosis: A descriptive study on clinical features and myopathology. Arthritis Res Ther. 2014;16:R111.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and serological hallmarks of systemic sclerosis overlap syndromes. J Rheumatol. 2011;38:2406-9.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of survival and causes of death in Japanese patients with systemic sclerosis. J Rheumatol. 2011;38:1931-9.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic sclerosis: Establishing diagnostic criteria Medicine (Baltimore) . 2010;89:159-65.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic sclerosis without antinuclear antibodies or Raynaud's phenomenon: A multicentre study in the prospective EULAR Scleroderma Trials and Research (EUSTAR) database. Rheumatology. 2013;52:560-7.
- [CrossRef] [PubMed] [Google Scholar]
- Subcutaneous calcinosis: Is it different between systemic sclerosis and dermatomyositis. ? J Scleroderma Relat Disord. 2022;7:7-23.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical subsets, skin thickness progression rate, and serum antibody levels in systemic sclerosis patients with anti-topoisomerase I antibody. Arthritis Rheum. 2007;56:2740.
- [CrossRef] [PubMed] [Google Scholar]
- Calcinosis is associated with ischemic manifestations and increased disability in patients with systemic sclerosis. Semin Arthritis Rheum. 2020;50:891-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin Rheumatol. 2014;33:1093.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac involvement in adult polymyositis or dermatomyositis: A systematic review. Clin Cardiol. 2012;35:686-91.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic sclerosis: Commonly asked questions by rheumatologists. J Clin Rheumatol. 2015;21:149-55.
- [CrossRef] [PubMed] [Google Scholar]
- Calcinosis is associated with digital ulcers and osteoporosis in patients with systemic sclerosis: A Scleroderma Clinical trials Consortium study. Semin Arthritis Rheum. 2016;46:344-9.
- [CrossRef] [PubMed] [Google Scholar]






