Translate this page into:
Amlodipine-induced gingival enlargement: A case report
*Corresponding author: Harish Kasarabada, Department of Internal Medicine, Army Hospital R & R, New Delhi, India. kasarabadaharish@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kasarabada H, Iyengar S, Jacob O, Reddy K. Amlodipine-induced gingival enlargement: A case report. Sri Ramachandra J Health Sci 2023;3:31-2.
Abstract
Drug-induced gingival overgrowth (DIGO) is a result of side effect of certain drugs. Among calcium channel blockers, gingival enlargement is more commonly seen with nifedipine. We present a rare case report of amlodipine-induced gingival enlargement which resolved after 4 weeks of stoppage of drug.
Keywords
Amlodipine
Drug-induced gingival overgrowth
Fibrotic gingival enlargement
INTRODUCTION
Hypertension is the leading risk factor for premature death and disability worldwide.[1] The most common ly prescribed anti-hypertensives are calcium channel blockers (CCBs). Among CCBs, Nefidipine is the most common drug to have side effect of drug-induced gingival overgrowth (DIGO). However, amlodipine a relatively new calcium blocker of dihydropyridine derivative is a long-acting drug with relatively fewer side effects. Amlodipine producing gingival enlargement is rare barring a few case reports.[2] It is noteworthy observation that gingival enlargement secondary to chronic usage of CCB is seen with L (Long-acting blockers of alpha 1 subtype of calcium channel) type dihydropyridine category. It has been observed that the drug-induced gingival enlargement is reversible and resolves following stoppage of responsible etiological drug. Antiepileptic drugs such as phenytoin, phenobarbitone, and immunosuppressants such as cyclosporine and tacrolimus are the other common causes of DIGO.[3]
CASE PRESENTATION
A 56-year-old woman was diagnosed as a case of primary hypertension 1 year before the onset gingival enlargement. She was started on amlodipine of 5 milligram (mg) dosage and was followed up regularly. She had good control of her blood pressure and showed good compliance to the drug. Following 6 months of her daily usage of amlodipine, she had developed progressive gingival overgrowth, initially was not symptomatic but with progressive gingival enlargement, she started to experience localized pain while brushing her teeth. In view of progression of symptoms, she approached our outpatient department. On examination, she had gingival overgrowth [Figure 1] and was referred to dental center. She returned with provisional diagnosis of fibrotic gingival enlargement which was grade 1 with gingival overgrowth restricted to interdental papillae, important differentials were DIGO and hereditary gingival overgrowth. She denied any family history of similar complaints; hence, DIGO diagnosis was made from exclusion and the responsible drug was thought to be amlodipine as there were few case reports suggesting gingival enlargement secondary to usage of CCB’s. As the provisional diagnosis of DIGO was made, the patient was not subjected to any oral prophylaxis like scaling. Amlodipine was stopped after explaining the side effect and taking her consent, in place of amlodipine angiotensin receptor blocker (ARB) Telmisartan in dosage of 40 mg was started. The rationale of starting ARB Telmisartan was as per physician choice following Joint National Committee (JNC) for hypertension eight guidelines. She was asked to follow-up regularly. Her blood pressure was within normal limits and after 3 months of stopping amlodipine gingival overgrowth was resolved and the patient was free of symptoms [Figure 2].
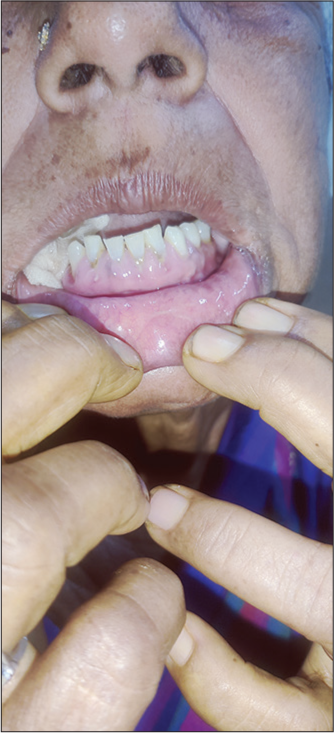
- Gingival enlargement following chronic usage of amlodipine.
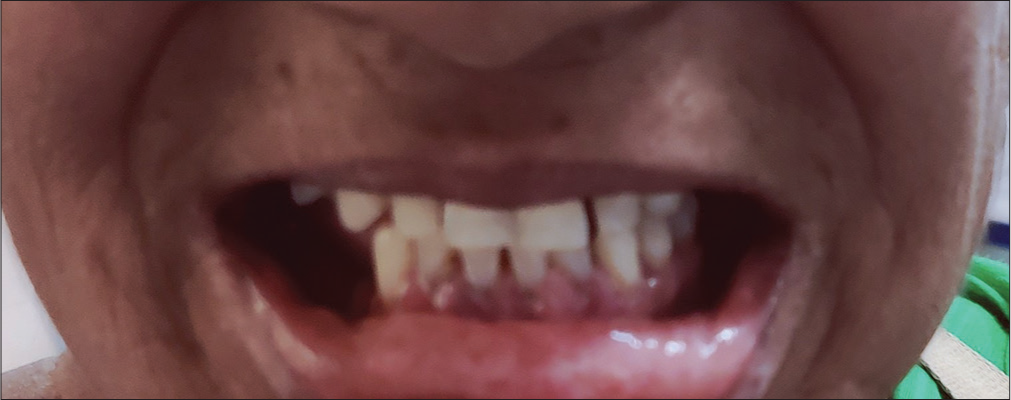
- Resolution to normal state after stoppage of amlodipine.
DISCUSSION
DIGO has been observed with many drugs and among CCB’s nifedipine was the most common drug. However, there are few case reports presenting DIGO secondary to chronic usage of amlodipine. Here, in our case, we have a similar case scenario where chronic usage of amlodipine leads to symptomatic gingival overgrowth which resolved on stoppage of the responsible etiological drug. Complete dental examination ruled out any possibility of bacterial infection, in view of the same scaling, was not performed and the responsible drug was stopped and advised patient for regular follow-up. The pathogenesis behind DIGO is not clear, the hypothetic proposed mechanism is believed to be accumulation of the drug in the gingival crevicular fluid in the presence of bacteria that can lead to upregulation of proinflammatory cytokines leading to gingival enlargement.[3] The accepted mechanism in CCB’s is that the inhibitory effects caused by CCB’s on cation channels leads to decreased folate intake by gingival fibroblasts. This will in turn lead to changes in local cytokine like tissue inhibitor of metalloproteinases-1 and matrix mettaloproteinase-1, resulting in failure in production of collagenase, leading to accumulation of connective tissue and finally resulting in DIGO.[4] Etiology of gingival enlargement apart from DIGO can be due to bacterial infections, vitamin C deficiency which results in inflammatory type of enlargement. Fibrotic gingival enlargement is exclusively seen with DIGO. In view of rarity of amlodipine usage leading to DIGO, we present this case.
CONCLUSION
Dentists and physicians should be aware about DIGO as it requires stoppage of responsible drug and change to other class of drug
Early identification at grade 0 or 1 can help in less morbidity and early resolution
Long-term hypertensive patients on CCB’s should be educated about the potential side effects and be advised to report to either dentist or physician at the earliest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223-37.
- [CrossRef] [PubMed] [Google Scholar]
- Gingival enlargement improvement following medication change from amlodipine to benidipine and periodontal therapy. BMJ Case Rep. 2022;15:e249879.
- [CrossRef] [PubMed] [Google Scholar]
- Amlodipine induced gingival enlargement. BMJ Case Rep. 2021;14:e245098.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanism of drug-induced gingival overgrowth revisited: A unifying hypothesis. Oral Dis. 2015;21:e51-61.
- [CrossRef] [PubMed] [Google Scholar]







