Translate this page into:
Serum magnesium levels in chronic kidney disease patients
*Corresponding author: Dr. M. Ganesh, Department of Biochemistry, Sri Ramachadra Medical College and Research Institute, Chennai, Tamil Nadu, India. hod.biochemistry@sriramachandra.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Kotha NB, Murugan G, Manikandan A, Selvarajan S, Muthukathan R. Serum magnesium levels in chronic kidney disease patients. Sri Ramachandra J Health Sci 2022;2:29-2.
Abstract
Objectives:
Chronic kidney disease (CKD) is a condition of life-threatening nature presenting with deterioration in kidney function which is both irreversible and progressive. It is characterized by progressive incapability of the kidneys to filter the excretory products of blood consequently necessitating dialysis to prevent azotemia, end-organ damage, and ultimately death. The role of the kidneys in the homeostasis of magnesium and conservation of this neglection ion’s concentration in blood is undeniable. The objective of the present study was to estimate serum magnesium in CKD subjects and healthy control groups.
Material and Methods:
Serum creatinine, eGFR, Serum magnesium was assesed in 37 CKD patients and 43 healthy controls.
Results:
The serum creatinine levels were significantly higher (P < 0.001) in cases (2.4 ± 0.88 [mg/dL]) when compared to healthy controls (0.85 ± 0.1 [mg/dL]) [Table 1]. The estimated GFR was calculated using the Modification of Diet in Renal Disease formula and was 31.2 ± 12.1 mL/min in cases and 85.67 ± 13.1 mL/min in controls and they were significantly different (P < 0.001). Serum magnesium was 2.02 ± 0.36 mg/dL in cases and 2.01 ± 0.17 mg/dL in controls.
Conclusion:
The overall distribution showed a trend of hypomagnesemia in CKD patients but it was not statistically significant (P = 0.877). In our study, though statistically trivial, we found a trend toward hypomagnesemia in CKD patients when compared to controls. The lack of significance could be because the CKD patients were not grouped according to stages. A larger study with proper categorization and exclusion can provide us with better insights into magnesium dynamics in CKD patients.
Keywords
Chronic kidney disease
Magnesium
Homeostasis
Hypermagnesemia
INTRODUCTION
Chronic kidney disease (CKD) is categorized among the leading causes of morbidity and mortality around the world. It is a global health-care burden with significantly higher rates of mortality, hospitalization, and health-care utilization. The prevalence of CKD is constantly increasing, with a worldwide prevalence of 8–13%. More than 40% of these affected individuals are dependent on renal replacement therapy like dialysis or renal transplant.[1] In addition, CKD is independently a risk factor of cardiovascular diseases and its related complications. Numerous literatures over the past years document the fact that the fatalities of advancing kidney disease are deferred or even prevented by timely diagnosis by means of laboratory testing.[2]
The kidney has a significant role to play in the homeostasis of magnesium and the conservation of serum concentration of this neglected ion. Magnesium holds a large shell of hydration; hence, the absorption, transport, and excretion are different when compared to similar divalent ions. However, the plasma distribution of magnesium is similar to calcium, with 55% in the ionized form, 15% in complexed form, and 30% in protein-bound form. Around 2400 mg of magnesium is generally filtered at the glomeruli every day and around 90% of the filtered load is normally reabsorbed. This reabsorption mechanism maintains the plasma magnesium concentrations whenever there is an excess or a deficit.[3] In patients with CKD, as the GFR falls, the filtered load of magnesium also falls. However, the reabsorption capacity remains fairly the same. This process ultimately leads to a picture of hypermagnesemia in CKD, especially in the later stages. However, in patients undergoing dialysis, the dialysate magnesium concentration plays a major role in determining the plasma magnesium concentrations post-dialysis.[4,5]
Maintenance of magnesium levels within the normal range is essential because hypomagnesemia is found to be associated with advancing cardiovascular incidents, calcification of the vessels, and impending morbidity.[6] Hence, in our study, we studied the levels of serum magnesium in CKD subjects compared to healthy controls.
MATERIAL AND METHODS
Study design
This study was a prospective and cross-sectional study conducted in the Biochemistry Department of SRMC&RI. The study was conducted in 2012 after approval from the Institute Ethics Committee (CSP/12/JAN/26/37). Our study consisted of two groups – Group 1: CKD patients and Group 2: Healthy controls. For Group 1, 37 CKD patients of all stages in both genders were included in the study. Patients having an associated cardiovascular disease, bone disorder, or alcoholism were excluded from the study. Patients taking medications for seizures, immunosuppressants, or steroids were also excluded as they may independently affect the serum magnesium levels. For Group 2, 43 healthy persons without any comorbidity of both genders were included in the study. Smokers, alcoholics, and persons with other comorbidities such as hypertension, diabetes, asthma, and any infectious diseases were excluded from the study.
Sample collection and processing
Blood samples were collected from the individuals in the random state using red-topped Vacutainer containing clot activators. The samples were centrifuged at 3500 rpm for a period of 10 min and then serum was separated and stored at −80°c until analysis. Magnesium was estimated in serum using the RXL dimension clinical chemistry system by the methyl thymol blue method. The values of serum magnesium are expressed as mg/ dL and the normal reference range is 1.8–2.4 mg/dL. Creatinine was estimated in serum by employing the kinetic Jaffe’s method. Estimated GFR (eGFR) was assessed from the serum creatinine concentration, by means of the Modification of Diet in Renal Disease (MDRD) study estimating equation – eGFR = 175 × S Cr −1.154 × age −0.203 × 1.212 [if black race] × 0.742 [if female].
Statistical analysis
Continuous variables (age, S. creatinine, eGFR, and S. magnesium) are expressed as mean and standard deviation. A comparison of these parameters between the two groups is done using the independent samples two-tailed t-test. P < 0.05 is considered to be statistically significant. SPSS software version 19.0 was utilized for statistical analysis. Graphs were constructed using Microsoft Excel 2016.
RESULTS
We analyzed the data from 37 cases (CKD patients) and 43 healthy controls. The mean age among the cases was 45 ± 10.5 whereas, in controls, it was 38.7 ± 7.8. The healthy controls were significantly younger when compared to cases (P = 0.003). The serum creatinine levels were significantly higher (P < 0.001) in cases (2.4 ± 0.88 [mg/dL]) when compared to healthy controls (0.85 ± 0.1 [mg/dL]) [Table 1]. The eGFR was calculated using the MDRD formula and was 31.2 ± 12.1 mL/ min in cases and 85.67 ± 13.1 mL/min in controls and they were significantly different (P < 0.001). Serum magnesium was 2.02 ± 0.36 mg/dL in cases and 2.01 ± 0.17 mg/dL in controls. The overall distribution showed a trend of hypomagnesemia in CKD patients but it was not statistically significant (P = 0.877) [Figure 1]. The hypomagnesemia was more on the lower side of normal rather than frank hypomagnesemia. In our data, there was 21.6% of frank hypomagnesemia and 10.8% of frank hypermagnesemia [Table 2].
| Parameter | Cases (n=37) | Controls (n=43) | P value |
|---|---|---|---|
| Age | 45±10.5 | 38.7±7.8 | 0.003* |
| S. creatinine (mg/dL) | 2.4±0.88 | 0.85±0.1 | <0.001* |
| eGFR (mL/min) | 31.2±12.1 | 85.67±13.1 | <0.001* |
| S. magnesium (mg/dL) |
2.02±0.36 | 2.01±0.17 | 0.877 |
Data represented as mean±standard deviation. Comparison done through two-tailed independent samples t-test as the test of significance. *Statistically significant (P<0.05). CKD: Chronic kidney disease, eGFR: Estimated glomerular filtration rate
| Magnesium levels | Number of cases | Percentage |
|---|---|---|
| Normal | 25 | 67.6 |
| Hypomagnesemia | 8 | 21.6 |
| Hypermagnesemia | 4 | 10.8 |
CKD: Chronic kidney disease
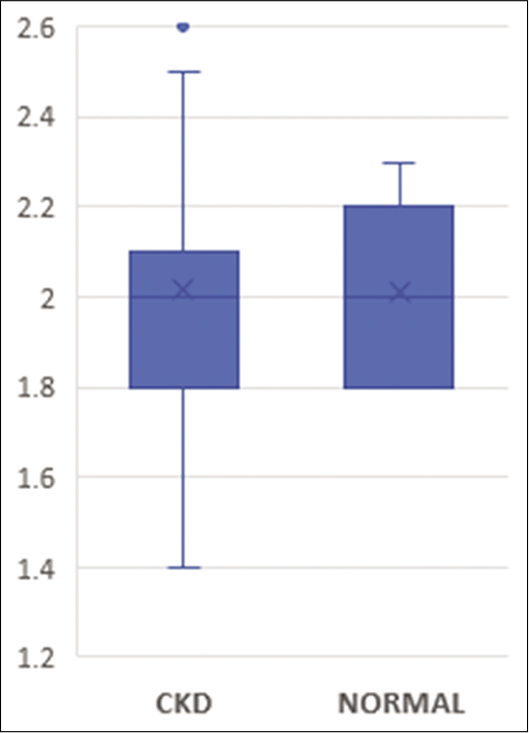
- Comparison of S. magnesium levels in CKD patients and healthy controls. CKD: Chronic kidney disease.
We also analyzed the gender-wise distribution of parameters within the CKD group. Serum magnesium had a slightly lower mean in males (2.01 ± 0.35 mg/dL) compared to females (2.03 ± 0.39 mg/dL) which were statistically insignificant (P = 0.874), but the overall distribution of values was trending on the higher side in males compared to the females [Figure 2 and Table 3].
| Parameter | Female (n=17) | Male (n=20) | P value |
|---|---|---|---|
| Age | 42±8.6 | 47.6±11.5 | 0.109 |
| S. creatinine (mg/dL) | 2.3±0.65 | 2.5±1.03 | 0.507 |
| eGFR (mL/min) | 28.5±14.5 | 33.4±9.3 | 0.228 |
| S. magnesium (mg/dL) | 2.03±0.39 | 2.01±0.35 | 0.874 |
Data represented as mean±standard deviation. Comparison done through two-tailed independent samples t-test as the test of significance. CKD: Chronic kidney disease, eGFR: Estimated glomerular filtration rate
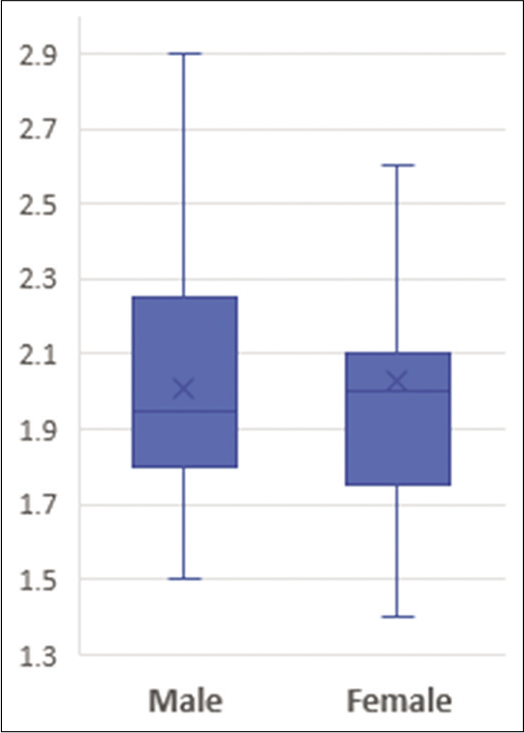
- Gender-wise distribution of S. magnesium in CKD patients. CKD: Chronic kidney disease.
DISCUSSION
Serum magnesium concentrations are dependent on appropriate renal function. Hence, in CKD patients, both hypo- and hypermagnesemia are fairly common.[7] Hypermagnesemia is commonly encountered in end-stage (Stage 4 and 5) renal disease because of extremely low GFR. On the other hand, hypomagnesemia is also often observed because of medications (diuretics, PO4 binders, antacids, and proton-pump inhibitors) and volume expansion.[5,8] In patients undergoing dialysis, the dialysate magnesium concentration also significantly affects the post-dialysis magnesium concentrations.[4] In one study, 10%–70% of the subjects on maintenance hemodialysis (HD) were found to have an increase in total body magnesium and an increasing occurrence of hypermagnesemia.[9] Incidence of hypermagnesemia is seen to vary in different cohorts of dialysis patients due to diversity in diet, changes in magnesium concentration in dialysis fluids, the methodology of measurement of magnesium, and the non-uniformity in the employed cutoff values. Certain literature state that with usage of dialysate fluid containing 0.5 mmol/L of magnesium, nearly 68% of the patients presented with hypermagnesemia.[10] Certain others claim that in all patients undergoing regular dialysis treatment, there is an increase in free magnesium concentrations.[4] Although despite such claims, the most of the subjects had only a very modest and clinically insignificant rise in magnesium. Since there is no correlation between serum magnesium levels and residual diuresis, it can be opined that the excretion of magnesium by cirrhotic kidneys is not pertinent for elimination of the ion. The restriction in elimination of magnesium in HD is because of its sluggish diffusion from the intracellular space, this action being similar to that of phosphates.[10,11] Therefore, to ensure efficient removal of magnesium in HD subjects, the only alternative is decreasing its concentration in the dialysate fluid.[12]
Decreased magnesium levels in serum are an independent prognosticator of mortality in dialysis subjects and are also increasingly correlated with common carotid arterial atherosclerosis.[5,6,13] There are numerous literature which also support the claim of protection against calcification of vessels with increasing magnesium concentration in dialysis patients. In vitro studies also prove that the presence of magnesium inhibits early growth of calcium phosphate crystals. Interaction of magnesium ions with metalloproteinase-2 may also affect the atherosclerotic process.[13] Some authors also suggest that magnesium supplementation has benefits beyond vascular protection and disease progression.[14]
CONCLUSION
In our study, though statistically trivial, we found a trend toward hypomagnesemia in CKD patients when compared to controls. The lack of significance could be because the CKD patients were not grouped according to stages. This could have resulted in an overlap of various CKD stages, nullifying any significant change. Furthermore, medications for treating CKD itself can directly affect the magnesium concentrations, acting as effect modifiers. A larger study with proper categorization and exclusion can provide us with better insights into magnesium dynamics in CKD patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global prevalence of chronic kidney disease a systematic review and meta-analysis. PLoS One. 2016;11:e0158765.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825-30.
- [CrossRef] [PubMed] [Google Scholar]
- Baseline serum magnesium level and its variability in maintenance hemodialysis patients: Associations with mortality In: Kidney Blood Press Res. Vol 44. 2019. p. :222-32.
- [CrossRef] [PubMed] [Google Scholar]
- Serum magnesium, mortality and disease progression in chronic kidney disease. BMC Nephrol. 2020;21:49.
- [CrossRef] [Google Scholar]
- Lower serum magnesium is associated with vascular calcification in peritoneal dialysis patients: A cross-sectional study. BMC Nephrol. 2017;18:129.
- [CrossRef] [PubMed] [Google Scholar]
- Magnesium in chronic kidney disease: Unanswered questions. Blood Purif. 2011;31:172-6.
- [CrossRef] [PubMed] [Google Scholar]
- Magnesium balance in chronic and end-stage kidney disease. Adv Chronic Kidney Dis. 2018;25:291-5.
- [CrossRef] [PubMed] [Google Scholar]
- Magnesium in chronic kidney disease: Should we care? Blood Purif. 2018;45:173-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dialysate potassium, dialysate magnesium, and hemodialysis risk. J Am Soc Nephrol. 2017;28:3441-51.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin Dial. 2015;28:564-77.
- [CrossRef] [PubMed] [Google Scholar]
- Magnesium in chronic kidney disease stages 3 and 4 and dialysis patients. Clin Kidney J. 2012;5:i39-51.
- [CrossRef] [PubMed] [Google Scholar]
- Magnesium: A magic bullet for cardiovascular disease in chronic kidney disease? Nutrients. 2019;11:E455.
- [CrossRef] [PubMed] [Google Scholar]
- Magnesium and progression of chronic kidney disease: Benefits beyond cardiovascular protection? Adv Chronic Kidney Dis. 2018;25:274-80.
- [CrossRef] [PubMed] [Google Scholar]






